Activation of PXR causes drug interactions with Paxlovid in transgenic mice
- PMID: 37969744
- PMCID: PMC10638548
- DOI: 10.1016/j.apsb.2023.08.001
Activation of PXR causes drug interactions with Paxlovid in transgenic mice
Abstract
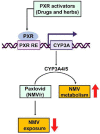
Paxlovid is a nirmatrelvir (NMV) and ritonavir (RTV) co-packaged medication used for the treatment of coronavirus disease 2019 (COVID-19). The active component of Paxlovid is NMV and RTV is a pharmacokinetic booster. Our work aimed to investigate the drug/herb-drug interactions associated with Paxlovid and provide mechanism-based guidance for the clinical use of Paxlovid. By using recombinant human cytochrome P450s (CYPs), we confirmed that CYP3A4 and 3A5 are the major enzymes responsible for NMV metabolism. The role of CYP3A in Paxlovid metabolism were further verified in Cyp3a-null mice, which showed that the deficiency of CYP3A significantly suppressed the metabolism of NMV and RTV. Pregnane X receptor (PXR) is a ligand-dependent transcription factor that upregulates CYP3A4/5 expression. We next explored the impact of drug- and herb-mediated PXR activation on Paxlovid metabolism in a transgenic mouse model expressing human PXR and CYP3A4/5. We found that PXR activation increased CYP3A4/5 expression, accelerated NMV metabolism, and reduced the systemic exposure of NMV. In summary, our work demonstrated that PXR activation can cause drug interactions with Paxlovid, suggesting that PXR-activating drugs and herbs should be used cautiously in COVID-19 patients receiving Paxlovid.
Keywords: CYP3A4/5; Drug/herb–drug interactions; Nirmatrelvir; Paxlovid; Pregnane X receptor; Rhynchophylline; Rifampicin; Ritonavir.
© 2023 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures






References
-
- Tsai P.H., Lai W.Y., Lin Y.Y., Luo Y.H., Lin Y.T., Chen H.K., et al. Clinical manifestation and disease progression in COVID-19 infection. J Chin Med Assoc. 2021;84:3–8. - PubMed
-
- Organization WH. WHO coronavirus (COVID-19), overview. Updated [2023/06/20]. Accessed [2023/06/29]. Available from: https://covid19.who.int/.
-
- Pfizer. Paxlovid. Accessed [2023/06/29]. Available from: https://www.paxlovid.com/.
Grants and funding
LinkOut - more resources
Full Text Sources

