Quantum Dots Assembled with Photosynthetic Antennae on a Carbon Nanotube Platform: A Nanohybrid for the Enhancement of Light Energy Harvesting
- PMID: 37969970
- PMCID: PMC10633852
- DOI: 10.1021/acsomega.3c07673
Quantum Dots Assembled with Photosynthetic Antennae on a Carbon Nanotube Platform: A Nanohybrid for the Enhancement of Light Energy Harvesting
Erratum in
-
Correction to "Quantum Dots Assembled with Photosynthetic Antennae on a Carbon Nanotube Platform: A Nanohybrid for the Enhancement of Light Energy Harvesting".ACS Omega. 2024 Jan 5;9(3):4137. doi: 10.1021/acsomega.3c09478. eCollection 2024 Jan 23. ACS Omega. 2024. PMID: 38284034 Free PMC article.
Abstract
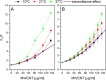
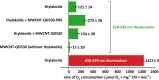
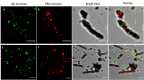
The construction of artificial systems for solar energy harvesting is still a challenge. There needs to be a light-harvesting antenna with a broad absorption spectrum and then the possibility to transfer harvested energy to the reaction center, converting photons into a storable form of energy. Bioinspired and bioderivative elements may help in achieving this aim. Here, we present an option for light harvesting: a nanobiohybrid of colloidal, semiconductor quantum dots (QDs) and natural photosynthetic antennae assembled on the surface of a carbon nanotube. For that, we used QDs of cadmium telluride and cyanobacterial phycobilisome rods (PBSr) or light-harvesting complex II (LHCII) of higher plants. For this nanobiohybrid, we confirmed composition and organization using infrared spectroscopy, X-ray photoelectron spectroscopy, and high-resolution confocal microscopy. Then, we proved that within such an assembly, there is a resonance energy transfer from QD to PBSr or LHCII. When such a nanobiohybrid was further combined with thylakoids, the energy was transferred to photosynthetic reaction centers and efficiently powered the photosystem I reaction center. The presented construct is proof of a general concept, combining interacting elements on a platform of a nanotube, allowing further variation within assembled elements.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Cohen-Ofri I.; van Gastel M.; Grzyb J.; Brandis A.; Pinkas I.; Lubitz W.; Noy D. Zinc-Bacteriochlorophyllide Dimers in de Novo Designed Four-Helix Bundle Proteins. A Model System for Natural Light Energy Harvesting and Dissipation. J. Am. Chem. Soc. 2011, 133, 9526–9535. 10.1021/ja202054m. - DOI - PubMed
LinkOut - more resources
Full Text Sources

