Kinetics, Equilibrium, and Thermodynamics for Conjugation of Chitosan with Insulin-Mimetic [ meso-Tetrakis(4-sulfonatophenyl)porphyrinato]oxovanadate(IV)(4-) in an Aqueous Solution
- PMID: 37970023
- PMCID: PMC10634234
- DOI: 10.1021/acsomega.3c05804
Kinetics, Equilibrium, and Thermodynamics for Conjugation of Chitosan with Insulin-Mimetic [ meso-Tetrakis(4-sulfonatophenyl)porphyrinato]oxovanadate(IV)(4-) in an Aqueous Solution
Abstract
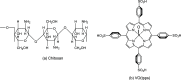
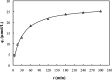
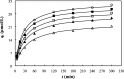
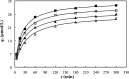
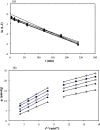
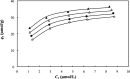
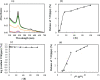
This study investigated the conjugation of chitosan with the insulin-mimetic [meso-tetrakis(4-sulfonatophenyl)porphyrinato]oxovanadate(IV)(4-), VO(tpps), in an aqueous medium as a function of conjugation time, VO(tpps) concentrations, and temperatures. To validate the synthesis of chitosan-VO(tpps) conjugate, UV-visible and Fourier transform infrared spectrophotometric techniques were utilized. Conjugate formation is ascribed to the electrostatic interaction between the NH3+ units of chitosan and the SO3- units of VO(tpps). Chitosan enhances the stability of VO(tpps) in an aqueous medium (pH 2.5). VO(tpps) conjugation with chitosan was best explained by pseudo-second-order kinetic and Langmuir isotherm models based on kinetic and isotherm studies. The Langmuir equation determined that the maximal ability of VO(tpps) conjugated with each gram of chitosan was 39.22 μmol at a solution temperature of 45 °C. Activation energy and thermodynamic studies (Ea: 8.78 kJ/mol, ΔG: -24.52 to -27.55 kJ/mol, ΔS: 204.22 J/(mol K), and ΔH: 37.30 kJ/mol) reveal that conjugation is endothermic and physical in nature. The discharge of VO(tpps) from conjugate was analyzed in freshly prepared 0.1 mol/L phosphate buffer (pH 7.4) at 37 °C. The release of VO(tpps) from the conjugate is a two-phase process best explained by the Higuchi model, according to a kinetic analysis of the release data. Taking into consideration all experimental findings, it is proposed that chitosan can be used to formulate both solid and liquid insulin-mimetic chitosan-VO(tpps) conjugates.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Carbon dioxide capture and conversion by an environmentally friendly chitosan based meso-tetrakis(4-sulfonatophenyl) porphyrin.Carbohydr Polym. 2017 Nov 1;175:575-583. doi: 10.1016/j.carbpol.2017.08.031. Epub 2017 Aug 11. Carbohydr Polym. 2017. PMID: 28917903
-
Improvement of hyperglycaemia and metabolic syndromes in type 2 diabetic KKAy mice by oral treatment with [meso-tetrakis(4-sulfonatophenyl) porphyrinato]oxovanadium(IV)(4-) complex.J Pharm Pharmacol. 2007 Mar;59(3):437-44. doi: 10.1211/jpp.59.3.0014. J Pharm Pharmacol. 2007. PMID: 17331348
-
pH-controlled self-assembling of meso-tetrakis(4-sulfonatophenyl)porphyrin-chitosan complexes.Biomacromolecules. 2009 May 11;10(5):1067-76. doi: 10.1021/bm8011715. Biomacromolecules. 2009. PMID: 19301895
-
Chitosan modification persimmon tannin bioadsorbent for highly efficient removal of Pb(II) from aqueous environment: the adsorption equilibrium, kinetics and thermodynamics.Environ Technol. 2019 Jan;40(1):112-124. doi: 10.1080/09593330.2017.1380712. Epub 2017 Oct 4. Environ Technol. 2019. PMID: 28911271
-
A [meso-tetrakis(4-sulfonatophenyl)porphyrinato]zinc(ii) complex as an oral therapeutic for the treatment of type 2 diabetic KKA(y) mice.ChemMedChem. 2007 Feb;2(2):218-25. doi: 10.1002/cmdc.200600228. ChemMedChem. 2007. PMID: 17245806
Cited by
-
Efficient adsorptive removal of levofloxacin using sulfonated graphene oxide: Adsorption behavior, kinetics, and thermodynamics.Heliyon. 2024 Nov 10;10(22):e40319. doi: 10.1016/j.heliyon.2024.e40319. eCollection 2024 Nov 30. Heliyon. 2024. PMID: 39641076 Free PMC article.
References
-
- Confederat L. G.; Motrescu I.; Condurache M. I.; Constantin S.; Bujor A.; Tuchilus C. G.; Profire L. Chitosan-based delivery systems loaded with glibenclamide and lipoic acid: Formulation, characterization, and kinetic release studies. Appl. Sci. 2020, 10, 7532.10.3390/app10217532. - DOI
-
- Constantin S. M.; Buron F.; Routier S.; Vasincu I. M.; Apotrosoaei M.; Lupascu F.; Confederat L.; Tuchilus C.; Constantin M. T.; Sava A.; Profire L. Formulation and characterization of new polymeric systems based on chitosan and xanthine derivatives with thiazolidin-4-one scaffold. Materials 2019, 12, 558.10.3390/ma12040558. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

