Improved Detection and Quantification of Cyclopropane Fatty Acids via Homonuclear Decoupling Double Irradiation NMR Methods
- PMID: 37970028
- PMCID: PMC10634279
- DOI: 10.1021/acsomega.3c06538
Improved Detection and Quantification of Cyclopropane Fatty Acids via Homonuclear Decoupling Double Irradiation NMR Methods
Abstract
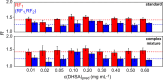
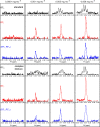
Over the years, NMR spectroscopy has become a powerful analytical tool for the identification and quantification of a variety of natural compounds in a broad range of food matrices. Furthermore, NMR can be useful for characterizing food matrices in terms of quality and authenticity, also allowing for the identification of counterfeits. Although NMR requires minimal sample preparation, this technique suffers from low intrinsic sensitivity relative to complementary techniques; thus, the detection of adulterants or markers for authenticity at low concentrations remains challenging. Here, we present a strategy to overcome this limitation by the introduction of a simple band-selective homonuclear decoupling sequence that consists of double irradiation on 1H during NMR signal acquisition. The utility of the proposed method is tested on dihydrosterculic acid (DHSA), one of the cyclopropane fatty acids (CPFAs) shown to be a powerful molecular marker for authentication of milk products. A quantitative description of how the proposed NMR scheme allows sensitivity enhancement yet accurate quantification of DHSA is provided.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Sobolev A. P.; Thomas F.; Donarski J.; Ingallina C.; Circi S.; Cesare Marincola F.; Capitani D.; Mannina L. Use of NMR Applications to Tackle Future Food Fraud Issues. Trends Food Sci. Technol. 2019, 91, 347–353. 10.1016/j.tifs.2019.07.035. - DOI
-
- Lytou A. E.; Panagou E. Z.; Nychas G.-J. E. Volatilomics for Food Quality and Authentication. Curr. Opin. Food Sci. 2019, 28, 88–95. 10.1016/j.cofs.2019.10.003. - DOI
-
- Román S.; Sánchez-Siles L. M.; Siegrist M. The Importance of Food Naturalness for Consumers: Results of a Systematic Review. Trends Food Sci. Technol. 2017, 67, 44–57. 10.1016/j.tifs.2017.06.010. - DOI
LinkOut - more resources
Full Text Sources

