Hydroxyapatite Incorporated with Fe3O4@MCM-41 Core-Shell: A Promising Nanocomposite for Teriparatide Delivery in Bone Tissue Regeneration
- PMID: 37970037
- PMCID: PMC10633862
- DOI: 10.1021/acsomega.3c04931
Hydroxyapatite Incorporated with Fe3O4@MCM-41 Core-Shell: A Promising Nanocomposite for Teriparatide Delivery in Bone Tissue Regeneration
Abstract
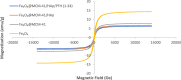
This article presents a comprehensive study of the development of a novel nanocomposite comprising core-shell Fe3O4@MCM-41 with superparamagnetic properties and hydroxyapatite (HAp). The nanocomposite serves as a pH-responsive nanocarrier, offering an efficient injectable dosage for teriparatide (PTH (1-34)) delivery. The aim is to address the limitations associated with drug-induced side effects, precautionary measures, and frequent injections. The nanocomposites, as prepared, were characterized using techniques including X-ray diffraction, Fourier transform infrared, zeta potential, dynamic light scattering, VSM, scanning electron microscopy, and transmission electron microscopy. The nanocomposites' average crystallite diameter was determined to be 27 ± 5 nm. The hydrodynamic size of the PTH (1-34)-loaded nanocarrier ranged from 357 to 495 nm, with a surface charge of -33 mV. The entrapment and loading efficiencies were determined to be 73% and 31%, respectively. All of these findings collectively affirm successful fabrication. Additionally, in vivo medication delivery was investigated using the HPLC method, mirroring the in vitro tests. Utilizing the dialysis approach, we demonstrated sustained-release behavior. PTH (1-34) diffusion increased as the pH decreased from 7.4 to 5.6. After 24 h, drug release was higher at acidic pH (88%) compared to normal pH (43%). The biocompatibility of the PTH (1-34)-loaded nanocarrier was assessed using the MTT assay employing the NIH3T3 and HEK-293 cell lines. The results demonstrated that the nanocarrier not only exhibited nontoxicity but also promoted cell proliferation and differentiation. In the in vivo test, the drug concentration reached 505 μg within 30 min of exposure to the magnetic field. Based on these findings, the Fe3O4@MCM-41/HAp/PTH (1-34) nanocomposite, in combination with a magnetic field, offers an efficient and biocompatible approach to enhance the therapeutic effect of osteogenesis and overcome drug limitations.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
Synthesis and characterization of a novel, pH-responsive sustained release nanocarrier using polyethylene glycol, graphene oxide, and natural silk fibroin protein by a green nano emulsification method to enhance cancer treatment.Int J Biol Macromol. 2023 Jan 31;226:1100-1115. doi: 10.1016/j.ijbiomac.2022.11.226. Epub 2022 Nov 24. Int J Biol Macromol. 2023. PMID: 36435465
-
Green synthesized polyvinylpyrrolidone/titanium dioxide hydrogel nanocomposite modified with agarose macromolecules for sustained and pH-responsive release of anticancer drug.Int J Biol Macromol. 2023 Jun 15;240:124345. doi: 10.1016/j.ijbiomac.2023.124345. Epub 2023 Apr 11. Int J Biol Macromol. 2023. PMID: 37054860
-
Novel Carboxymethyl Cellulose-Based Hydrogel with Core-Shell Fe3O4@SiO2 Nanoparticles for Quercetin Delivery.Materials (Basel). 2022 Dec 7;15(24):8711. doi: 10.3390/ma15248711. Materials (Basel). 2022. PMID: 36556516 Free PMC article.
-
pH-Responsible Doxorubicin-Loaded Fe3O4@CaCO3 Nanocomposites for Cancer Treatment.Pharmaceutics. 2023 Feb 26;15(3):771. doi: 10.3390/pharmaceutics15030771. Pharmaceutics. 2023. PMID: 36986632 Free PMC article.
-
Mesopourous Fe3O4@SiO2-hydroxyapatite nanocomposite: Green sonochemical synthesis using strawberry fruit extract as a capping agent, characterization and their application in sulfasalazine delivery and cytotoxicity.J Hazard Mater. 2020 Dec 5;400:123140. doi: 10.1016/j.jhazmat.2020.123140. Epub 2020 Jun 11. J Hazard Mater. 2020. PMID: 32563904
References
-
- Jones M. S.; Waterson B. Principles of management of long bone fractures and fracture healing. Surgery 2020, 38 (2), 91–99. 10.1016/j.mpsur.2019.12.010. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

