Influence of Fatty Acid Modification on the Anticancer Activity of the Antimicrobial Peptide Figainin 1
- PMID: 37970064
- PMCID: PMC10633881
- DOI: 10.1021/acsomega.3c06806
Influence of Fatty Acid Modification on the Anticancer Activity of the Antimicrobial Peptide Figainin 1
Abstract
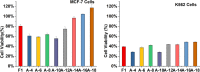
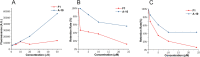
Antimicrobial peptides derived from the skin secretions of amphibians have made important progress in tumor therapy due to their unique mechanism of destroying cell membranes. Figainin 1 (F1) is an 18-amino acid antimicrobial peptide from the skin secretions of Boana raniceps frogs. In a previous study, F1 was shown to inhibit cancer cell proliferation. F1 is composed entirely of natural amino acids; therefore, it is easily degraded by a variety of proteases, resulting in poor stability and a short half-life. In the present study, we used a fatty acid modification strategy to improve the stability of Figainin 1. Among the 8 peptides synthesized, A-10 showed the strongest antiproliferative activity against K562 cells and the other four tumor cell lines, and its stability against serum and proteinase K was improved compared with F1. We found that A-10 works through two mechanisms, cell membrane destruction and apoptosis, and can arrest the cell cycle in the G0/G1 phase. Moreover, A-10 exhibited self-assembly behavior. Overall, it is necessary to select a fatty acid with a suitable length for modification to improve the stability and antiproliferative activity of antimicrobial peptides. This study provides a good reference for the development of antimicrobial peptides as effective anticancer compounds.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
LinkOut - more resources
Full Text Sources

