Urine Quantification Following Furosemide for Severe Acute Kidney Injury Prediction in Critically Ill Children
- PMID: 37970140
- PMCID: PMC10631834
- DOI: 10.1055/s-0041-1732447
Urine Quantification Following Furosemide for Severe Acute Kidney Injury Prediction in Critically Ill Children
Abstract
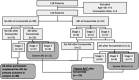
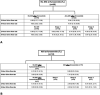
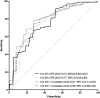
A standardized, quantified assessment of furosemide responsiveness predicts acute kidney injury (AKI) in children after cardiac surgery and AKI progression in critically ill adults. The purpose of this study was to determine if response to furosemide is predictive of severe AKI in critically ill children outside of cardiac surgery. We performed a multicenter retrospective study of critically ill children. Quantification of furosemide response was based on urine flow rate (normalized for weight) measurement 0 to 6 hours after the dose. The primary outcome was presence of creatinine defined severe AKI (Kidney Disease Improving Global Outcomes stage 2 or greater) within 7 days of furosemide administration. Secondary outcomes included mortality, duration of mechanical ventilation and length of stay. A total of 110 patients were analyzed. Severe AKI occurred in 20% ( n = 22). Both 2- and 6-hour urine flow rate were significantly lower in those with severe AKI compared with no AKI ( p = 0.002 and p < 0.001). Cutoffs for 2- and 6-hour urine flow rate for prediction of severe AKI were <4 and <3 mL/kg/hour, respectively. The adjusted odds of developing severe AKI for 2-hour urine flow rate of <4 mL/kg/hour was 4.3 (95% confidence interval [CI]: 1.33-14.15; p = 0.02). The adjusted odds of developing severe AKI for 6-hour urine flow rate of <3 mL/kg/hour was 6.19 (95% CI: 1.85-20.70; p = 0.003). Urine flow rate in response to furosemide is predictive of severe AKI in critically ill children. A prospective assessment of urine flow rate in response to furosemide for predicting subsequent severe AKI is warranted.
Keywords: acute kidney injury; critically ill; furosemide; pediatrics; urine flow rate.
Thieme. All rights reserved.
Conflict of interest statement
Conflict of interest None declared.
Figures
References
-
- Rewa O, Bagshaw S M. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(04):193–207. - PubMed
-
- Assessment of Worldwide Acute Kidney Injury, Renal Angina and, Epidemiology (AWARE) Investigators . Kaddourah A, Basu R K, Goldstein S L, Sutherland S M. Oliguria and acute kidney injury in critically ill children: implications for diagnosis and outcomes. Pediatr Crit Care Med. 2019;20(04):332–339. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

