Regulation of apoptosis by ubiquitination in liver cancer
- PMID: 37970337
- PMCID: PMC10636691
Regulation of apoptosis by ubiquitination in liver cancer
Abstract
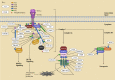
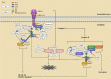
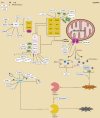
Apoptosis is a programmed cell death process critical to cell development and tissue homeostasis in multicellular organisms. Defective apoptosis is a crucial step in the malignant transformation of cells, including hepatocellular carcinoma (HCC), where the apoptosis rate is higher than in normal liver tissues. Ubiquitination, a post-translational modification process, plays a precise role in regulating the formation and function of different death-signaling complexes, including those involved in apoptosis. Aberrant expression of E3 ubiquitin ligases (E3s) in liver cancer (LC), such as cellular inhibitors of apoptosis proteins (cIAPs), X chromosome-linked IAP (XIAP), and linear ubiquitin chain assembly complex (LUBAC), can contribute to HCC development by promoting cell survival and inhibiting apoptosis. Therefore, the review introduces the main apoptosis pathways and the regulation of proteins in these pathways by E3s and deubiquitinating enzymes (DUBs). It summarizes the abnormal expression of these regulators in HCC and their effects on cancer inhibition or promotion. Understanding the role of ubiquitination in apoptosis and LC can provide insights into potential targets for therapeutic intervention.
Keywords: Apoptosis; E3s; hepatocellular carcinoma; ubiquitin.
AJCR Copyright © 2023.
Conflict of interest statement
None.
Figures
Similar articles
-
Biochemistry, Pathophysiology, and Regulation of Linear Ubiquitination: Intricate Regulation by Coordinated Functions of the Associated Ligase and Deubiquitinase.Cells. 2021 Oct 9;10(10):2706. doi: 10.3390/cells10102706. Cells. 2021. PMID: 34685685 Free PMC article. Review.
-
Targeting Ferroptosis by Ubiquitin System Enzymes: A Potential Therapeutic Strategy in Cancer.Int J Biol Sci. 2022 Aug 29;18(14):5475-5488. doi: 10.7150/ijbs.73790. eCollection 2022. Int J Biol Sci. 2022. PMID: 36147464 Free PMC article. Review.
-
Ubiquitin signaling in pancreatic ductal adenocarcinoma.Front Mol Biosci. 2023 Dec 20;10:1304639. doi: 10.3389/fmolb.2023.1304639. eCollection 2023. Front Mol Biosci. 2023. PMID: 38174069 Free PMC article. Review.
-
The HOIL-1L ligase modulates immune signalling and cell death via monoubiquitination of LUBAC.Nat Cell Biol. 2020 Jun;22(6):663-673. doi: 10.1038/s41556-020-0517-9. Epub 2020 May 11. Nat Cell Biol. 2020. PMID: 32393887
-
The Effect of Dysfunctional Ubiquitin Enzymes in the Pathogenesis of Most Common Diseases.Int J Mol Sci. 2020 Sep 1;21(17):6335. doi: 10.3390/ijms21176335. Int J Mol Sci. 2020. PMID: 32882786 Free PMC article. Review.
Cited by
-
Analysis and identification of PTBP2 as an oncogene in hepatocellular carcinoma.Discov Oncol. 2025 Apr 12;16(1):520. doi: 10.1007/s12672-025-02216-9. Discov Oncol. 2025. PMID: 40220253 Free PMC article.
-
Deciphering Aflatoxin B1 affected critical molecular pathways governing cancer: A bioinformatics study using CTD and PANTHER databases.Mycotoxin Res. 2025 Feb;41(1):93-111. doi: 10.1007/s12550-024-00563-0. Epub 2024 Oct 17. Mycotoxin Res. 2025. PMID: 39417919
-
Ubiquitination in hepatocellular carcinoma immunity.J Transl Med. 2025 May 23;23(1):574. doi: 10.1186/s12967-025-06592-2. J Transl Med. 2025. PMID: 40410880 Free PMC article. Review.
-
Strategic advancement of E3 ubiquitin ligase in the management of hepatocellular carcinoma.Med Oncol. 2024 Jun 18;41(7):178. doi: 10.1007/s12032-024-02411-8. Med Oncol. 2024. PMID: 38888684 Review.
-
Green-synthesized selenium-hydroxytyrosol nanocomposites attenuate hepatocellular carcinoma in rats by modulating oxidative stress, inflammation, and apoptosis.Naunyn Schmiedebergs Arch Pharmacol. 2025 Mar 27. doi: 10.1007/s00210-025-04034-w. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40146247
References
-
- Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL, Rebrikov D, Brodianski VM, Kemper OC, Kollet O, Lapidot T, Soffer D, Sobe T, Avraham KB, Goncharov T, Holtmann H, Lonai P, Wallach D. Targeted disruption of the mouse caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. - PubMed
-
- Wan Y, Yang L, Jiang S, Qian D, Duan J. Excessive apoptosis in ulcerative colitis: crosstalk between apoptosis, ROS, ER stress, and Intestinal homeostasis. Inflamm Bowel Dis. 2022;28:639–648. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
