Lemon flavonoids nutraceutical (Eriomin®) attenuates prediabetes intestinal dysbiosis: A double-blind randomized controlled trial
- PMID: 37970408
- PMCID: PMC10630820
- DOI: 10.1002/fsn3.3654
Lemon flavonoids nutraceutical (Eriomin®) attenuates prediabetes intestinal dysbiosis: A double-blind randomized controlled trial
Abstract
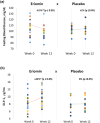
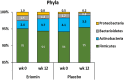
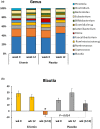
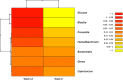
Eriocitrin (eriodictyol 7-O-β-rutinoside), a citrus flavonoid from lemon juice and peel, reduces hyperglycemia and improves diabetes-related biomarkers in prediabetes patients. Eriocitrin is first metabolized by gut microbiota, producing energy for gut cells and short chain fatty acids that play a relevant role in glycemic control. The aim of this study was to assess the effect of Eriomin®, a nutraceutical composed of 70% eriocitrin, 5% hesperidin, and 4% naringin, on the microbiota of prediabetic patients. Patients were randomly divided into two groups and received unlabeled capsules of Eriomin® (200 mg/day) or placebo during 12 weeks. After treatment with the nutraceutical, it was a 6% decrease of hyperglycemia and 22% increase of GLP-1 blood levels of (p < .05). The profile of intestinal microorganisms, obtained by 16S rRNA sequencing of the patients' feces extract, showed changes in microbiota composition, such as lower growth of Firmicutes and less abundance of the Lachnospiraceae family. The family Ruminococcaceae increased and Blautia genus reduced with Eriomin® supplementation. In additional, Blautia was positively correlated with hyperglycemia reduction. In conclusion, the nutraceutical Eriomin® moderately reduced the growth of microorganisms associated with intestinal dysbiosis and increased the abundance of beneficial bacteria. Changes promoted mainly by the flavonoid eriocitrin in the microbiota were related to a lower glycemic level and increased production of GLP-1 in patients with prediabetes.
Keywords: Eriomin®; clinical trial; eriocitrin nutraceutical; intestinal dysbiosis; lemon flavonoid; microbiota (16S rRNA); pre‐diabetes.
© 2023 The Authors. Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Figures


References
-
- American Diabetes Association Professional Practice Committee , Draznin, B. , Aroda, V. R. , Bakris, G. , Benson, G. , Brown, F. M. , Freeman, R. , Green, J. , Huang, E. , Isaacs, D. , Kahan, S. , Leon, J. , Lyons, S. K. , Peters, A. L. , Prahalad, P. , Reusch, J. E. B. , Young‐Hyman, D. , Das, S. , & Kosiborod, M. (2022). Classification and diagnosis of diabetes: Standards of medical care in diabetes‐2022. Diabetes Care, 45, S17–S38. 10.2337/dc22-S002 - DOI - PubMed
-
- Amiot, M. J. , Riva, C. , & Vinet, A. (2016). Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obesity Reviews, 17, 573–586. - PubMed
-
- Benítez‐Páez, A. , Gómez Del Pugar, E. M. , López‐Almela, I. , Moya‐Pérez, Á. , Codoñer‐Franch, P. , & Sanz, Y. (2020). Depletion of Blautia species in the microbiota of obese children relates to intestinal inflammation and metabolic phenotype worsening. mSystems, 5(2), e00857. 10.1128/mSystems.00857-19 - DOI - PMC - PubMed

