Interaction between host cell proteins and open reading frames of porcine circovirus type 2
- PMID: 37970506
- PMCID: PMC10640953
- DOI: 10.5187/jast.2023.e67
Interaction between host cell proteins and open reading frames of porcine circovirus type 2
Abstract
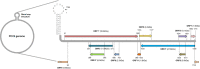
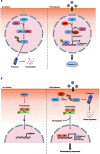
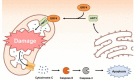
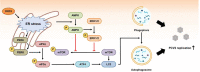
Postweaning multisystemic wasting syndrome (PMWS) is caused by a systemic inflammation after porcine circovirus type 2 (PCV2) infection. It was one of the most economically important pathogens affecting pig production worldwide before PCV2 vaccine was first introduced in 2006. After the development of a vaccine against PCV2a type, pig farms gradually restored enormous economic losses from PMWS. However, vaccine against PCV2a type could not be fully effective against several different PCV2 genotypes (PCV2b - PCV2h). In addition, PCV2a vaccine itself could generate antigenic drift of PCV2 capsid. Therefore, PCV2 infection still threats pig industry worldwide. PCV2 infection was initially found in local tissues including reproductive, respiratory, and digestive tracks. However, PCV2 infection often leads to a systemic inflammation which can cause severe immunosuppression by depleting peripheral lymphocytes in secondary lymphoid tissues. Subsequently, a secondary infection with other microorganisms can cause PMWS. Eleven putative open reading frames (ORFs) have been predicted to encode PCV2 genome. Among them, gene products of six ORFs from ORF1 to ORF6 have been identified and characterized to estimate its functional role during PCV2 infection. Acquiring knowledge about the specific interaction between each PCV2 ORF protein and host protein might be a key to develop preventive or therapeutic tools to control PCV2 infection. In this article, we reviewed current understanding of how each ORF of PCV2 manipulates host cell signaling related to immune suppression caused by PCV2.
Keywords: Host cell protein; Open reading frame; Pathogenesis; Pig; Porcine circovirus type 2.
© Copyright 2023 Korean Society of Animal Science and Technology.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

