CAR T-cell Design-dependent Remodeling of the Brain Tumor Immune Microenvironment Modulates Tumor-associated Macrophages and Anti-glioma Activity
- PMID: 37971169
- PMCID: PMC10689147
- DOI: 10.1158/2767-9764.CRC-23-0424
CAR T-cell Design-dependent Remodeling of the Brain Tumor Immune Microenvironment Modulates Tumor-associated Macrophages and Anti-glioma Activity
Abstract
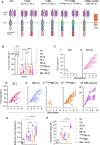
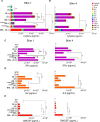
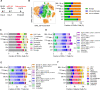
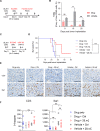
Understanding the intricate dynamics between adoptively transferred immune cells and the brain tumor immune microenvironment (TIME) is crucial for the development of effective T cell-based immunotherapies. In this study, we investigated the influence of the TIME and chimeric antigen receptor (CAR) design on the anti-glioma activity of B7-H3-specific CAR T-cells. Using an immunocompetent glioma model, we evaluated a panel of seven fully murine B7-H3 CARs with variations in transmembrane, costimulatory, and activation domains. We then investigated changes in the TIME following CAR T-cell therapy using high-dimensional flow cytometry and single-cell RNA sequencing. Our results show that five out of six B7-H3 CARs with single costimulatory domains demonstrated robust functionality in vitro. However, these CARs had significantly varied levels of antitumor activity in vivo. To enhance therapeutic effectiveness and persistence, we incorporated 41BB and CD28 costimulation through transgenic expression of 41BBL on CD28-based CAR T-cells. This CAR design was associated with significantly improved anti-glioma efficacy in vitro but did not result in similar improvements in vivo. Analysis of the TIME revealed that CAR T-cell therapy influenced the composition of the TIME, with the recruitment and activation of distinct macrophage and endogenous T-cell subsets crucial for successful antitumor responses. Indeed, complete brain macrophage depletion using a CSF1R inhibitor abrogated CAR T-cell antitumor activity. In sum, our study highlights the critical role of CAR design and its modulation of the TIME in mediating the efficacy of adoptive immunotherapy for high-grade glioma.
Significance: CAR T-cell immunotherapies hold great potential for treating brain cancers; however, they are hindered by a challenging immune environment that dampens their effectiveness. In this study, we show that the CAR design influences the makeup of the immune environment in brain tumors, underscoring the need to target specific immune components to improve CAR T-cell performance, and highlighting the significance of using models with functional immune systems to optimize this therapy.
© 2023 The Authors; Published by the American Association for Cancer Research.
Figures



Update of
-
CAR T-cell design dependent remodeling of the brain tumor immune microenvironment identify macrophages as key players that inhibit or promote anti-tumor activity.Res Sq [Preprint]. 2023 Jun 6:rs.3.rs-2972427. doi: 10.21203/rs.3.rs-2972427/v1. Res Sq. 2023. Update in: Cancer Res Commun. 2023 Dec 1;3(12):2430-2446. doi: 10.1158/2767-9764.CRC-23-0424. PMID: 37333156 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

