Optimizing dosing of the cycloserine pro-drug terizidone in children with rifampicin-resistant tuberculosis
- PMID: 37971239
- PMCID: PMC10720412
- DOI: 10.1128/aac.00611-23
Optimizing dosing of the cycloserine pro-drug terizidone in children with rifampicin-resistant tuberculosis
Abstract
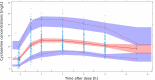
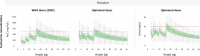
There are no pharmacokinetic data in children on terizidone, a pro-drug of cycloserine and a World Health Organization (WHO)-recommended group B drug for rifampicin-resistant tuberculosis (RR-TB) treatment. We collected pharmacokinetic data in children <15 years routinely receiving 15-20 mg/kg of daily terizidone for RR-TB treatment. We developed a population pharmacokinetic model of cycloserine assuming a 2-to-1 molecular ratio between terizidone and cycloserine. We included 107 children with median (interquartile range) age and weight of 3.33 (1.55, 5.07) years and 13.0 (10.1, 17.0) kg, respectively. The pharmacokinetics of cycloserine was described with a one-compartment model with first-order elimination and parallel transit compartment absorption. Allometric scaling using fat-free mass best accounted for the effect of body size, and clearance displayed maturation with age. The clearance in a typical 13 kg child was estimated at 0.474 L/h. The mean absorption transit time when capsules were opened and administered as powder was significantly faster compared to when capsules were swallowed whole (10.1 vs 72.6 min) but with no effect on bioavailability. Lower bioavailability (-16%) was observed in children with weight-for-age z-score below -2. Compared to adults given 500 mg daily terizidone, 2022 WHO-recommended pediatric doses result in lower exposures in weight bands 3-10 kg and 36-46 kg. We developed a population pharmacokinetic model in children for cycloserine dosed as terizidone and characterized the effects of body size, age, formulation manipulation, and underweight-for-age. With current terizidone dosing, pediatric cycloserine exposures are lower than adult values for several weight groups. New optimized dosing is suggested for prospective evaluation.
Keywords: Mycobacterium tuberculosis; antibiotic resistance; pediatric drug therapy; pharmacokinetics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Steady-state population pharmacokinetics of terizidone and its metabolite cycloserine in patients with drug-resistant tuberculosis.Br J Clin Pharmacol. 2019 Sep;85(9):1946-1956. doi: 10.1111/bcp.13975. Epub 2019 Jul 12. Br J Clin Pharmacol. 2019. PMID: 31046167 Free PMC article.
-
Population Pharmacokinetics of Cycloserine and Pharmacokinetic/Pharmacodynamic Target Attainment in Multidrug-Resistant Tuberculosis Patients Dosed with Terizidone.Antimicrob Agents Chemother. 2020 Oct 20;64(11):e01381-20. doi: 10.1128/AAC.01381-20. Print 2020 Oct 20. Antimicrob Agents Chemother. 2020. PMID: 32816738 Free PMC article.
-
Levofloxacin Population Pharmacokinetics in South African Children Treated for Multidrug-Resistant Tuberculosis.Antimicrob Agents Chemother. 2018 Jan 25;62(2):e01521-17. doi: 10.1128/AAC.01521-17. Print 2018 Feb. Antimicrob Agents Chemother. 2018. PMID: 29133560 Free PMC article.
-
d-Cycloserine Pharmacokinetics/Pharmacodynamics, Susceptibility, and Dosing Implications in Multidrug-resistant Tuberculosis: A Faustian Deal.Clin Infect Dis. 2018 Nov 28;67(suppl_3):S308-S316. doi: 10.1093/cid/ciy624. Clin Infect Dis. 2018. PMID: 30496460 Free PMC article.
-
Pharmacokinetics of Second-Line Anti-Tubercular Drugs.Indian J Pediatr. 2019 Aug;86(8):714-716. doi: 10.1007/s12098-019-02923-6. Epub 2019 Mar 28. Indian J Pediatr. 2019. PMID: 30924070 Review.
Cited by
-
The Adverse Effects of Tuberculosis Treatment: A Comprehensive Literature Review.Medicina (Kaunas). 2025 May 17;61(5):911. doi: 10.3390/medicina61050911. Medicina (Kaunas). 2025. PMID: 40428869 Free PMC article. Review.
References
-
- 2022. WHO operational handbook on tuberculosis. Module 5: management of tuberculosis in children and adolescents. World Health Organization, Geneva. - PubMed
-
- 2022. WHO consolidated guidelines on tuberculosis. Module 5: management of tuberculosis in children and adolescents. World Health Organization, Geneva. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

