Subcutaneous infusion of high-dose benzathine penicillin G is safe, tolerable, and suitable for less-frequent dosing for rheumatic heart disease secondary prophylaxis: a phase 1 open-label population pharmacokinetic study
- PMID: 37971244
- PMCID: PMC10720493
- DOI: 10.1128/aac.00962-23
Subcutaneous infusion of high-dose benzathine penicillin G is safe, tolerable, and suitable for less-frequent dosing for rheumatic heart disease secondary prophylaxis: a phase 1 open-label population pharmacokinetic study
Abstract
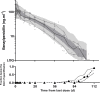
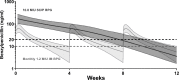
Since 1955, the recommended strategy for rheumatic heart disease (RHD) secondary prophylaxis has been benzathine penicillin G [BPG; 1.2 MU (900 mg)] injections administered intramuscularly every 4 weeks. Due to dosing frequency, pain, and programmatic challenges, adherence is suboptimal. It has previously been demonstrated that BPG delivered subcutaneously at a standard dose is safe and tolerable and has favorable pharmacokinetics, setting the scene for improved regimens with less frequent administration. The safety, tolerability, and pharmacokinetics of subcutaneous infusions of high-dose BPG were assessed in 24 healthy adult volunteers assigned to receive either 3.6, 7.2, or 10.8 MU (three, six, and nine times the standard dose, respectively) as a single subcutaneous infusion. The delivery of the BPG to the subcutaneous tissue was confirmed with ultrasonography. Safety assessments, pain scores, and penicillin concentrations were measured for 16 weeks post-dose. Subcutaneous infusion of penicillin (SCIP) was generally well tolerated with all participants experiencing transient, mild infusion-site reactions. Prolonged elevated penicillin concentrations were described using a combined zero-order (44 days) and first-order (t1/2 = 12 days) absorption pharmacokinetic model. In simulations, time above the conventionally accepted target concentration of 20 ng/mL (0.02 µg/mL) was 57 days for 10.8 MU delivered by subcutaneous infusion every 13 weeks compared with 9 days of every 4-weekly dosing interval for the standard 1.2 MU intramuscular dose (i.e., 63% and 32% of the dosing interval, respectively). High-dose SCIP (BPG) is safe, has acceptable tolerability, and may be suitable for up to 3 monthly dosing intervals for secondary prophylaxis of RHD.
Keywords: benzathine penicillin G; population pharmacokinetics; rheumatic heart disease; secondary antibiotic prophylaxis; subcutaneous infusions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Oliver J, Bennett J, Thomas S, Zhang J, Pierse N, Moreland NJ, Williamson DA, Jack S, Baker M. 2021. Preceding group A Streptococcus skin and throat infections are individually associated with acute rheumatic fever: evidence from New Zealand. BMJ Glob Health 6:e007038. doi: 10.1136/bmjgh-2021-007038 - DOI - PMC - PubMed
-
- Katzenellenbogen JM, Bond-Smith D, Seth RJ, Dempsey K, Cannon J, Stacey I, Wade V, de Klerk N, Greenland M, Sanfilippo FM, Brown A, Carapetis JR, Wyber R, Nedkoff L, Hung J, Bessarab D, Ralph AP. 2020. Contemporary incidence and prevalence of rheumatic fever and rheumatic heart disease in Australia using linked data: the case for policy change. J Am Heart Assoc 9:e016851. doi: 10.1161/JAHA.120.016851 - DOI - PMC - PubMed
-
- Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. 2020. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 7012/Cure Kids (CK)
- StrepA PhD Scholarship, Scholarship for International Research Fees/University of Western Australia (UWA)
- Post Graduate Research Scholarship (Athelstan Saw Bequest Fund)/University of Western Australia (UWA)
- Research Program Training Scholarship/University of Western Australia (UWA)
- Wesfarmers Centre of Vaccines and Infectious Diseases Top Up Scholarship/Telethon Kids Institute (TKI)
LinkOut - more resources
Full Text Sources
Research Materials

