Maternal preconception stress produces sex-specific effects at the maternal:fetal interface to impact offspring development and phenotypic outcomes†
- PMID: 37971364
- PMCID: PMC10873277
- DOI: 10.1093/biolre/ioad156
Maternal preconception stress produces sex-specific effects at the maternal:fetal interface to impact offspring development and phenotypic outcomes†
Abstract
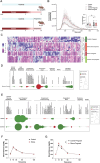
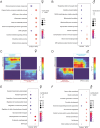
Entering pregnancy with a history of adversity, including adverse childhood experiences and racial discrimination stress, is a predictor of negative maternal and fetal health outcomes. Little is known about the biological mechanisms by which preconception adverse experiences are stored and impact future offspring health outcomes. In our maternal preconception stress (MPS) model, female mice underwent chronic stress from postnatal days 28-70 and were mated 2 weeks post-stress. Maternal preconception stress dams blunted the pregnancy-induced shift in the circulating extracellular vesicle proteome and reduced glucose tolerance at mid-gestation, suggesting a shift in pregnancy adaptation. To investigate MPS effects at the maternal:fetal interface, we probed the mid-gestation placental, uterine, and fetal brain tissue transcriptome. Male and female placentas differentially regulated expression of genes involved in growth and metabolic signaling in response to gestation in an MPS dam. We also report novel offspring sex- and MPS-specific responses in the uterine tissue apposing these placentas. In the fetal compartment, MPS female offspring reduced expression of neurodevelopmental genes. Using a ribosome-tagging transgenic approach we detected a dramatic increase in genes involved in chromatin regulation in a PVN-enriched neuronal population in females at PN21. While MPS had an additive effect on high-fat-diet (HFD)-induced weight gain in male offspring, both MPS and HFD were necessary to induce significant weight gain in female offspring. These data highlight the preconception period as a determinant of maternal health in pregnancy and provides novel insights into mechanisms by which maternal stress history impacts offspring developmental programming.
Keywords: PVN; developmental programming; extracellular vesicles; placenta; preconception stress; sex-specific; uterus.
© The Author(s) 2023. Published by Oxford University Press on behalf of Society for the Study of Reproduction. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures




Similar articles
-
Seminal plasma uterine priming alters uterine transcriptomics and negatively impacts embryo growth and uterine artery resistance but not offspring liver transcriptomics in beef cattle.J Anim Sci. 2024 Jan 3;102:skae300. doi: 10.1093/jas/skae300. J Anim Sci. 2024. PMID: 39367540
-
Effects of rate of body weight gain during the first trimester of gestation on beef heifer and offspring performance, concentrations of hormones and metabolites, and response to vaccination.J Anim Sci. 2024 Jan 3;102:skae193. doi: 10.1093/jas/skae193. J Anim Sci. 2024. PMID: 39028632 Free PMC article.
-
Methionine supply during mid-gestation modulates the bovine placental mTOR pathway, nutrient transporters, and offspring birth weight in a sex-specific manner.J Anim Sci. 2024 Jan 3;102:skae305. doi: 10.1093/jas/skae305. J Anim Sci. 2024. PMID: 39390894
-
Maternal and neonatal outcomes of elective induction of labor.Evid Rep Technol Assess (Full Rep). 2009 Mar;(176):1-257. Evid Rep Technol Assess (Full Rep). 2009. PMID: 19408970 Free PMC article.
-
Placental Exosomes During Gestation: Liquid Biopsies Carrying Signals for the Regulation of Human Parturition.Curr Pharm Des. 2018;24(9):974-982. doi: 10.2174/1381612824666180125164429. Curr Pharm Des. 2018. PMID: 29376493 Free PMC article. Review.
Cited by
-
Association of placental mitochondrial DNA mutations on infant negative affectivity: modifying effects of maternal lifetime stress and infant sex.Biol Sex Differ. 2025 Jun 10;16(1):40. doi: 10.1186/s13293-025-00717-4. Biol Sex Differ. 2025. PMID: 40495250 Free PMC article.
-
Trauma and Sensory Systems: Biological Mechanisms Involving the Skin and the 17q21 Gene Cluster.Biol Psychiatry. 2025 May 1;97(9):854-861. doi: 10.1016/j.biopsych.2024.11.003. Epub 2024 Nov 7. Biol Psychiatry. 2025. PMID: 39521032 Review.
-
The microbiome as a modulator of neurological health across the maternal-offspring interface.J Clin Invest. 2025 Feb 17;135(4):e184314. doi: 10.1172/JCI184314. J Clin Invest. 2025. PMID: 39959974 Free PMC article. Review.
-
Association of Placental Mitochondrial DNA Mutations on Infant Negative Affectivity: Modifying Effects of Maternal Lifetime Stress and Infant Sex.Res Sq [Preprint]. 2025 May 6:rs.3.rs-5806105. doi: 10.21203/rs.3.rs-5806105/v1. Res Sq. 2025. Update in: Biol Sex Differ. 2025 Jun 10;16(1):40. doi: 10.1186/s13293-025-00717-4. PMID: 40386437 Free PMC article. Updated. Preprint.
References
-
- Racine N, Plamondon A, Madigan S, McDonald S, Tough S. Maternal adverse childhood experiences and infant development. Pediatrics 2018; 141:e20172495. - PubMed
-
- Letourneau N, Dewey D, Kaplan BJ, Ntanda H, Novick J, Thomas JC, Deane AJ, Leung B, Pon K, Giesbrecht GF, the APrON Study Team . Intergenerational transmission of adverse childhood experiences via maternal depression and anxiety and moderation by child sex. J Dev Orig Health Dis 2019; 10:88–99. - PubMed
-
- Racine NM, Madigan SL, Plamondon AR, McDonald SW, Tough SC. Differential associations of adverse childhood experience on maternal health. Am J Prev Med 2018; 54:368–375. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

