Cost Savings and Waste Reduction Through Redispensing Unused Oral Anticancer Drugs: The ROAD Study
- PMID: 37971730
- PMCID: PMC10654927
- DOI: 10.1001/jamaoncol.2023.4865
Cost Savings and Waste Reduction Through Redispensing Unused Oral Anticancer Drugs: The ROAD Study
Erratum in
-
Change in email address.JAMA Oncol. 2025 Feb 1;11(2):189. doi: 10.1001/jamaoncol.2024.6289. JAMA Oncol. 2025. PMID: 39699887 Free PMC article. No abstract available.
Abstract
Importance: New strategies targeting waste are required to improve financial and ecologic sustainability of expensive therapies, such as oral anticancer drugs, that frequently remain unused by patients. Redispensing unused oral anticancer drugs seems to be a promising strategy when drug quality is guaranteed.
Objectives: To determine the waste reduction and net cost savings attained by redispensing oral anticancer drugs that go unused by patients compared with the standard practice of disposal.
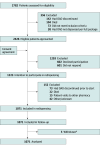
Design, settings, and participants: The ROAD study was a prospective single-group intervention conducted in the outpatient pharmacies of 4 hospitals in the Netherlands from February 1, 2021, to February 1, 2023, with 12-month follow-up of each patient. Patients with cancer and who had a prescription for an oral anticancer drug that could be stored at room temperature were included. Of 2426 eligible patients, 602 did not consent and 601 did not respond. Data analyses were performed from August 25, 2022, to April 19, 2023.
Intervention: Participants received oral anticancer drugs for use at home in special packaging (ie, sealed packaging with time-temperature indicator), to be returned to the pharmacy should these remain unused. The pharmacy ensured quality of returned drugs based on authenticity, appearance, remaining shelf life and adequate storage temperature. Drugs fulfilling quality requirements were redispensed to other patients.
Main outcome and measure: Total waste reduction and mean net annual cost savings per patient compared with the standard practice of disposal. Optimization of cost savings was explored by introducing variations in the quality assurance procedure and patient population. All analyses used the average exchange rate for 2021 €1 = US $1.18.
Results: Of 1223 patients with cancer who consented, 1071 participated (median [IQR] age, 70 [62-75] years; 622 [58.1%] were male). In all, 171 patients (16.0%; 95% CI, 13.8%-18.3%) returned 335 unused oral anticancer drug packages. Of the returned drugs, 228 packages were redispensed, which reduced waste by 68.1% (95% CI, 67.7%-68.5%) compared with the standard practice (disposal). Redispensing unused oral anticancer drugs comprised 2.4% (95% CI, 2.2%-2.5%) of total drug costs, providing mean net annual cost savings of US $680 (95% CI, $524-$837) up to $1591 (95% CI, $1226-$2002) per participant.
Conclusions and relevance: The findings of this multicenter intervention study indicate that redispensing unused oral anticancer drugs is associated with waste reduction and cost savings, which in turn may improve the affordability and sustainability of cancer treatment.
Trial registration: World Health Organization International Clinical Trials Registry Platform Identifier: NL9208.
Conflict of interest statement
Figures
References
-
- Organisation for Economic Co-operation and Development Health Policy Studies . Pharmaceutical Innovation and Access to Medicines. 2018. Accessed October 16, 2023. doi: 10.1787/9789264307391-en - DOI
-
- Smale EM, Egberts TCG, Heerdink ER, van den Bemt BJF, Bekker CL. Waste-minimising measures to achieve sustainable supply and use of medication. Sustain Chem Pharm. 2021;20:100400. doi: 10.1016/j.scp.2021.100400 - DOI
-
- National Academies of Sciences, Engineering and Medicine, Health and Medicine Division, Board on Health Care Services, Committee on Ensuring Patient Access to Affordable Drug Therapies . Making Medicines Affordable: A National Imperative. National Academies Press; 2017. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous