Project harmony: A systematic review and network meta-analysis of psychotherapy and pharmacologic trials for comorbid posttraumatic stress, alcohol, and other drug use disorders
- PMID: 37971855
- PMCID: PMC10939977
- DOI: 10.1037/bul0000409
Project harmony: A systematic review and network meta-analysis of psychotherapy and pharmacologic trials for comorbid posttraumatic stress, alcohol, and other drug use disorders
Abstract
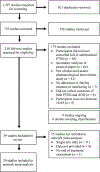
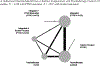
We conducted a systematic review and network meta-analyses (NMA) of psychotherapy and pharmacologic treatments for individuals with co-occurring posttraumatic stress disorder (PTSD) and alcohol or other drug use disorder (AOD). A comprehensive search spanning 1995-2019 yielded a pool of 39 studies for systematic review, including 24 randomized controlled trials for the NMA. Study interventions were grouped by target of treatment (PTSD + AOD, PTSD-only, and AOD-only) and approach (psychotherapy or medication). Standardized mean differences (SMD) from the NMA yielded evidence that at the end of treatment, integrated, trauma-focused therapy for PTSD + AOD was more effective at reducing PTSD symptoms than integrated, non-trauma-focused therapy (SMD = -0.30), AOD-focused psychotherapy (SMD = -0.29), and other control psychotherapies (SMD = -0.43). End-of-treatment alcohol use severity was less for AOD medication compared to placebo medication (SMD = -0.36) and trauma-focused therapy for PTSD + placebo medication (SMD = -0.67), and less for trauma-focused psychotherapy + AOD medication compared to PTSD medication (SMD = -0.53), placebo medication (SMD = -0.50), and trauma-focused psychotherapy + placebo medication (SMD = -0.81). Key limitations include the small number of studies in the NMA for pharmacologic treatments and the lack of demographic diversity apparent in the existing literature. Findings suggest room for new studies that can address limitations in study sample composition, sample sizes, retention, and apply new techniques for conducting comparative effectiveness in PTSD + AOD treatment. (PsycInfo Database Record (c) 2024 APA, all rights reserved).
Conflict of interest statement
Drs. Sudie Back and Therese Killeen disclose conflicts of interest as they are authors on the COPE therapy manuals published by Oxford University Press. All other authors report no conflicts of interest.
Figures












Similar articles
-
Psychological and pharmacological interventions for posttraumatic stress disorder and comorbid mental health problems following complex traumatic events: Systematic review and component network meta-analysis.PLoS Med. 2020 Aug 19;17(8):e1003262. doi: 10.1371/journal.pmed.1003262. eCollection 2020 Aug. PLoS Med. 2020. PMID: 32813696 Free PMC article.
-
Psychological therapies for the treatment of mental disorders in low- and middle-income countries affected by humanitarian crises.Cochrane Database Syst Rev. 2018 Jul 5;7(7):CD011849. doi: 10.1002/14651858.CD011849.pub2. Cochrane Database Syst Rev. 2018. PMID: 29975811 Free PMC article.
-
Pharmacotherapy for posttraumatic stress disorder.Cochrane Database Syst Rev. 2000;(4):CD002795. doi: 10.1002/14651858.CD002795. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2006 Jan 25;(1):CD002795. doi: 10.1002/14651858.CD002795.pub2. PMID: 11034765 Updated.
-
Early pharmacological interventions for universal prevention of post-traumatic stress disorder (PTSD).Cochrane Database Syst Rev. 2022 Feb 10;2(2):CD013443. doi: 10.1002/14651858.CD013443.pub2. Cochrane Database Syst Rev. 2022. PMID: 35141873 Free PMC article.
-
Psychological treatment of post-traumatic stress disorder (PTSD).Cochrane Database Syst Rev. 2007 Jul 18;(3):CD003388. doi: 10.1002/14651858.CD003388.pub3. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2013 Dec 13;(12):CD003388. doi: 10.1002/14651858.CD003388.pub4. PMID: 17636720 Updated.
Cited by
-
[Evidence-based psychotherapy of posttraumatic stress syndrome-An update].Nervenarzt. 2024 Jul;95(7):616-621. doi: 10.1007/s00115-024-01694-6. Epub 2024 Jun 21. Nervenarzt. 2024. PMID: 38906997 Review. German.
-
Integrating care for chronic pain and PTSD: A qualitative exploration of staff and patient perspectives.Psychol Serv. 2025 May 5:10.1037/ser0000968. doi: 10.1037/ser0000968. Online ahead of print. Psychol Serv. 2025. PMID: 40323858
-
The role of recurrent trauma on post-traumatic stress disorder symptoms and substance use among trauma exposed youth.Psychiatry Res. 2024 Aug;338:115980. doi: 10.1016/j.psychres.2024.115980. Epub 2024 May 27. Psychiatry Res. 2024. PMID: 38833935 Free PMC article.
-
Concurrent Treatment of Posttraumatic Stress Disorder and Alcohol Use Disorder in Women: A Randomized Clinical Trial.JAMA Netw Open. 2025 Jul 1;8(7):e2521087. doi: 10.1001/jamanetworkopen.2025.21087. JAMA Netw Open. 2025. PMID: 40663349 Free PMC article. Clinical Trial.
-
Design and methodology of the first open-label trial of MDMA-assisted therapy for veterans with post-traumatic stress disorder and alcohol use disorder: Considerations for a randomized controlled trial.Contemp Clin Trials Commun. 2024 Jul 20;41:101333. doi: 10.1016/j.conctc.2024.101333. eCollection 2024 Oct. Contemp Clin Trials Commun. 2024. PMID: 39262902 Free PMC article.
References
-
- Alegría M, Fortuna LR, Lin JY, Norris LF, Gao S, Takeuchi DT, Jackson JS Shrout PE, & Valentine A (2013). Prevalence, risk, and correlates of posttraumatic stress disorder across ethnic and racial minority groups in the U.S. Medical Care, 51(12), 1114–1123. 10.1097/MLR.0000000000000007 - DOI - PMC - PubMed
-
- Back SE, McCauley JL, Korte KJ, Gros DF, Leavitt V, Gray KM, Hamner MB, DeSantis SM, Malcolm R, Brady KT, & Kalivas PW (2016). A double-blind randomized controlled pilot trial of N-Acetylcysteine in veterans with PTSD and substance use disorders. The Journal of Clinical Psychiatry, 77(11), e1439–e1446. 10.4088/JCP.15m10239 - DOI - PMC - PubMed
-
- Back SE, Killeen T, Badour CL, Flanagan JC, Allan NP, Ana ES, Lozano B, Korte KJ, Foa EB, & Brady KT (2019). Concurrent treatment of substance use disorders and PTSD using prolonged exposure: A randomized clinical trial in military veterans. Addictive Behaviors, 90, 369–377. 10.1016/j.addbeh.2018.11.032 - DOI - PMC - PubMed

