Efficient pulmonary lymphatic drainage is necessary for inflammation resolution in ARDS
- PMID: 37971881
- PMCID: PMC10906459
- DOI: 10.1172/jci.insight.173440
Efficient pulmonary lymphatic drainage is necessary for inflammation resolution in ARDS
Abstract
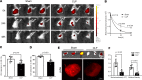
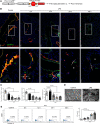
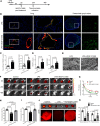
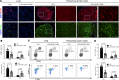
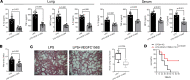
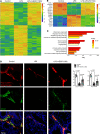
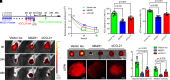
The lymphatic vasculature is the natural pathway for the resolution of inflammation, yet the role of pulmonary lymphatic drainage function in sepsis-induced acute respiratory distress syndrome (ARDS) remains poorly characterized. In this study, indocyanine green-near infrared lymphatic living imaging was performed to examine pulmonary lymphatic drainage function in septic mouse models. We found that the pulmonary lymphatic drainage was impaired owing to the damaged lymphatic structure in sepsis-induced ARDS. Moreover, prior lymphatic defects by blocking vascular endothelial growth factor receptor-3 (VEGFR-3) worsened sepsis-induced lymphatic dysfunction and inflammation. Posttreatment with vascular endothelial growth factor-C (Cys156Ser) (VEGF-C156S), a ligand of VEGFR-3, ameliorated lymphatic drainage by rejuvenating lymphatics to reduce the pulmonary edema and promote draining of pulmonary macrophages and neutrophils to pretracheal lymph nodes. Meanwhile, VEGF-C156S posttreatment reversed sepsis-inhibited CC chemokine ligand 21 (CCL21), which colocalizes with pulmonary lymphatic vessels. Furthermore, the advantages of VEGF-C156S on the drainage of inflammatory cells and edema fluid were abolished by blocking VEGFR-3 or CCL21. These results suggest that efficient pulmonary lymphatic drainage is necessary for inflammation resolution in ARDS. Our findings offer a therapeutic approach to sepsis-induced ARDS by promoting lymphatic drainage function.
Keywords: Pulmonology; Respiration; Vascular Biology.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

