Female sex protects against renal edema, but not lung edema, in mice with partial deletion of the endothelial barrier regulator Tie2 compared to male sex
- PMID: 37972011
- PMCID: PMC10653528
- DOI: 10.1371/journal.pone.0293673
Female sex protects against renal edema, but not lung edema, in mice with partial deletion of the endothelial barrier regulator Tie2 compared to male sex
Abstract
Background: The endothelial angiopoietin/Tie2 system is an important regulator of endothelial permeability and targeting Tie2 reduces hemorrhagic shock-induced organ edema in males. However, sexual dimorphism of the endothelium has not been taken into account. This study investigated whether there are sex-related differences in the endothelial angiopoietin/Tie2 system and edema formation.
Methods: Adult male and female heterozygous Tie2 knockout mice (Tie2+/-) and wild-type controls (Tie2+/+) were included (n = 9 per group). Renal and pulmonary injury were determined by wet/dry weight ratio and H&E staining of tissue sections. Protein levels were studied in plasma by ELISA and pulmonary and renal mRNA expression levels by RT-qPCR.
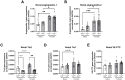
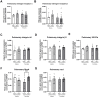
Results: In Tie2+/+ mice, females had higher circulating angiopoietin-2 (138%, p<0.05) compared to males. Gene expression of angiopoietin-1 (204%, p<0.01), angiopoietin-2 (542%, p<0.001) were higher in females compared to males in kidneys, but not in lungs. Gene expression of Tie2, Tie1 and VE-PTP were similar between males and females in both organs. Renal and pulmonary wet/dry weight ratio did not differ between Tie2+/+ females and males. Tie2+/+ females had lower circulating NGAL (41%, p<0.01) compared to males, whereas renal NGAL and KIM1 gene expression was unaffected. Interestingly, male Tie2+/- mice had 28% higher renal wet/dry weight ratio (p<0.05) compared to Tie2+/+ males, which was not observed in females nor in lungs. Partial deletion of Tie2 did not affect circulating angiopoietin-1 or angiopoietin-2, but soluble Tie2 was 44% and 53% lower in males and females, respectively, compared to Tie2+/+ mice of the same sex. Renal and pulmonary gene expression of angiopoietin-1, angiopoietin-2, estrogen receptors and other endothelial barrier regulators was comparable between Tie2+/- and Tie2+/+ mice in both sexes.
Conclusion: Female sex seems to protect against renal, but not pulmonary edema in heterozygous Tie2 knock-out mice. This could not be explained by sex dimorphism in the endothelial angiopoietin/Tie2 system.
Copyright: © 2023 van Leeuwen et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Lopez MC, Efron PA, Ozrazgat-Baslanti T, Zhang J, Cuschieri J, Maier RV, et al.. Sex-based differences in the genomic response, innate immunity, organ dysfunction, and clinical outcomes after severe blunt traumatic injury and hemorrhagic shock. J Trauma Acute Care Surg. 2016;81:478–85. doi: 10.1097/TA.0000000000001113 - DOI - PMC - PubMed
-
- Sperry JL, Nathens AB, Frankel HL, Vanek SL, Moore EE, Maier RV, et al.. Inflammation and the Host Response to Injury Investigators. Characterization of the gender dimorphism after injury and hemorrhagic shock: are hormonal differences responsible? Crit Care Med. 2008;36:1838–45. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

