p53-armed oncolytic adenovirus induces autophagy and apoptosis in KRAS and BRAF-mutant colorectal cancer cells
- PMID: 37972012
- PMCID: PMC10653454
- DOI: 10.1371/journal.pone.0294491
p53-armed oncolytic adenovirus induces autophagy and apoptosis in KRAS and BRAF-mutant colorectal cancer cells
Abstract
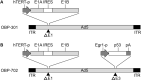
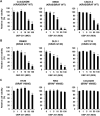
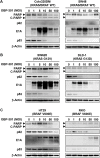
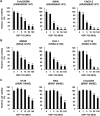
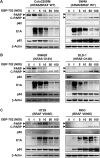
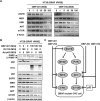
Colorectal cancer (CRC) cells harboring KRAS or BRAF mutations show a more-malignant phenotype than cells with wild-type KRAS and BRAF. KRAS/BRAF-wild-type CRCs are sensitive to epidermal growth factor receptor (EGFR)-targeting agents, whereas KRAS/BRAF-mutant CRCs are resistant due to constitutive activation of the EGFR-downstream KRAS/BRAF signaling pathway. Novel therapeutic strategies to treat KRAS/BRAF mutant CRC cells are thus needed. We recently demonstrated that the telomerase-specific replication-competent oncolytic adenoviruses OBP-301 and p53-armed OBP-702 exhibit therapeutic potential against KRAS-mutant human pancreatic cancer cells. In this study, we evaluated the therapeutic potential of OBP-301 and OBP-702 against human CRC cells with differing KRAS/BRAF status. Human CRC cells with wild-type KRAS/BRAF (SW48, Colo320DM, CACO-2), mutant KRAS (DLD-1, SW620, HCT116), and mutant BRAF (RKO, HT29, COLO205) were used in this study. The antitumor effect of OBP-301 and OBP-702 against CRC cells was analyzed using the XTT assay. Virus-mediated modulation of apoptosis, autophagy, and the EGFR-MEK-ERK and AKT-mTOR signaling pathways was analyzed by Western blotting. Wild-type and KRAS-mutant CRC cells were sensitive to OBP-301 and OBP-702, whereas BRAF-mutant CRC cells were sensitive to OBP-702 but resistant to OBP-301. Western blot analysis demonstrated that OBP-301 induced autophagy and that OBP-702 induced autophagy and apoptosis in human CRC cells. In BRAF-mutant CRC cells, OBP-301 and OBP-702 suppressed the expression of EGFR, MEK, ERK, and AKT proteins, whereas mTOR expression was suppressed only by OBP-702. Our results suggest that p53-armed oncolytic virotherapy is a viable therapeutic option for treating KRAS/BRAF-mutant CRC cells via induction of autophagy and apoptosis.
Copyright: © 2023 Tamura et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Y.U. is the President & CEO of Oncolys BioPharma, Inc. H.T. and T.F. are consultants for Oncolys BioPharma, Inc. The other authors have no potential conflicts of interest to disclose. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
Similar articles
-
BRAF associated autophagy exploitation: BRAF and autophagy inhibitors synergise to efficiently overcome resistance of BRAF mutant colorectal cancer cells.Oncotarget. 2016 Feb 23;7(8):9188-221. doi: 10.18632/oncotarget.6942. Oncotarget. 2016. PMID: 26802026 Free PMC article.
-
Relationships among KRAS mutation status, expression of RAS pathway signaling molecules, and clinicopathological features and prognosis of patients with colorectal cancer.World J Gastroenterol. 2019 Feb 21;25(7):808-823. doi: 10.3748/wjg.v25.i7.808. World J Gastroenterol. 2019. PMID: 30809081 Free PMC article.
-
Concomitant inhibition of receptor tyrosine kinases and downstream AKT synergistically inhibited growth of KRAS/BRAF mutant colorectal cancer cells.Oncotarget. 2017 Jan 17;8(3):5003-5015. doi: 10.18632/oncotarget.14009. Oncotarget. 2017. PMID: 28002807 Free PMC article.
-
Dual Inhibition of MEK and PI3K Pathway in KRAS and BRAF Mutated Colorectal Cancers.Int J Mol Sci. 2015 Sep 23;16(9):22976-88. doi: 10.3390/ijms160922976. Int J Mol Sci. 2015. PMID: 26404261 Free PMC article. Review.
-
Recommendations from the EGAPP Working Group: can testing of tumor tissue for mutations in EGFR pathway downstream effector genes in patients with metastatic colorectal cancer improve health outcomes by guiding decisions regarding anti-EGFR therapy?Genet Med. 2013 Jul;15(7):517-27. doi: 10.1038/gim.2012.184. Epub 2013 Feb 21. Genet Med. 2013. PMID: 23429431
Cited by
-
Safety profile of sikamat virus and its oncolytic potential in leukemic cells and cancer stem cells.Sci Rep. 2025 Apr 22;15(1):13817. doi: 10.1038/s41598-025-96061-z. Sci Rep. 2025. PMID: 40258869 Free PMC article.
-
Differential Cytotoxicity of Surface-Functionalized Silver Nanoparticles in Colorectal Cancer and Ex-Vivo Healthy Colonocyte Models.Cancers (Basel). 2025 Apr 27;17(9):1475. doi: 10.3390/cancers17091475. Cancers (Basel). 2025. PMID: 40361403 Free PMC article.
References
-
- Garcia-Carbonero N, Martinez-Useros J, Li W, Orta A, Perez N, Carames C, et al.. KRAS and BRAF Mutations as Prognostic and Predictive Biomarkers for Standard Chemotherapy Response in Metastatic Colorectal Cancer: A Single Institutional Study. Cells. 2020;9(1):219. Epub 2020/01/19. doi: 10.3390/cells9010219 ; PubMed Central PMCID: PMC7016634. - DOI - PMC - PubMed
-
- Ros J, Balconi F, Baraibar I, Saoudi Gonzalez N, Salva F, Tabernero J, et al.. Advances in immune checkpoint inhibitor combination strategies for microsatellite stable colorectal cancer. Front Oncol. 2023;13:1112276. Epub 2023/02/24. doi: 10.3389/fonc.2023.1112276 ; PubMed Central PMCID: PMC9932591. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

