Differential Viral Dynamics by Sex and Body Mass Index During Acute SARS-CoV-2 Infection: Results From a Longitudinal Cohort Study
- PMID: 37972270
- PMCID: PMC11093673
- DOI: 10.1093/cid/ciad701
Differential Viral Dynamics by Sex and Body Mass Index During Acute SARS-CoV-2 Infection: Results From a Longitudinal Cohort Study
Abstract
Background: There is evidence of an association of severe coroanavirus disease (COVID-19) outcomes with increased body mass index (BMI) and male sex. However, few studies have examined the interaction between sex and BMI on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral dynamics.
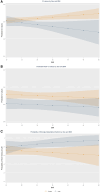
Methods: Participants conducted RT-PCR testing every 24-48 hours over a 15-day period. Sex and BMI were self-reported, and Ct values from E-gene were used to quantify viral load. Three distinct outcomes were examined using mixed-effects generalized linear models, linear models, and logistic models, respectively: all Ct values (model 1), nadir Ct value (model 2), and strongly detectable infection (at least 1 Ct value ≤28 during their infection) (model 3). An interaction term between BMI and sex was included, and inverse logit transformations were applied to quantify the differences by BMI and sex using marginal predictions.
Results: In total, 7988 participants enrolled in this study and 439 participants (model 1) and 309 (models 2 and 3) were eligible for these analyses. Among males, increasing BMI was associated with lower Ct values in a dose-response fashion. For participants with BMIs greater than 29 kg/m2, males had significantly lower Ct values and nadir Ct values than females. In total, 67.8% of males and 55.3% of females recorded a strongly detectable infection; increasing proportions of men had Ct values <28 with BMIs of 35 and 40 kg/m2.
Conclusions: We observed sex-based dimorphism in relation to BMI and COVID-19 viral load. Further investigation is needed to determine the cause, clinical impact, and transmission implications of this sex-differential effect of BMI on viral load.
Keywords: Ct value; SARS-CoV-2; biological sex; body mass index; viral load.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. V. K. is principal, and T. S. and E. H. are employees of, the healthcare technology company CareEvolution, which was contracted to configure the smartphone study app, provide operational and logistical support, and collaborate on the overall research approach. L. V. R. is an employee of Quest Diagnostics LLC, which was contracted to provide direct-to-consumer kits, logistical support for RT-PCR testing, and operational support for producing molecular testing results (but were blinded to the symptoms of the participants and the assays were analyzed according to existing manual of operating procedures). D. D. M. reports consulting and research grants from Bristol-Myers Squibb and Pfizer, consulting and research support from Fitbit, consulting and research support from Flexcon, a research grant from Boehringer Ingelheim, consulting from Avania, nonfinancial research support from Apple Computer, and consulting/other support from Heart Rhythm Society. Y. C. M. has received tests from Quanterix, Becton-Dickinson, Ceres, and Hologic for research-related purposes; consults for Abbott on subjects unrelated to SARS-CoV-2; and receives funding support to Johns Hopkins University from miDiagnostics. Y. C. M. also reports grants or contracts to their institution from the Centers for Disease Control and Prevention (CDC), NIH, and the Henry Jackson Foundation; a leadership role on the Infectious Diseases Institute Board; and receipt of equipment, materials, drugs, medical writing, gifts, or other services from Hologic, Cepheid, Roche, and ChemBio. H. L. reports grants or contracts from the NIH (U01AG068221-01A1), Alzheimer's Association (AARG-NTF-20-643020), American Heart Association (20SFRN35460034), and European Commission (847770) and consulting fees paid to the author from the University of Texas Health San Antonio and Headwaters Innovation, Inc. N. H. reports participation on Supporting Treatment Access and Recovery through Linkage Support (STAR-LS) (Principal Investigator: David Smelson; funding: CDC; Data and Safety Monitoring Board [DSMB] member). K. L. reports grants or contracts from Moderna (Correlates of EBV Immune Protection or Disease; CT-mRNA-1273-P203, CT-mRNA-1273-P204, KL2TR01455, mRNA-1273-P206/CT202204791), NIH/National Institute of Allergy and Infectious Diseases (NIAID) (1UM1 AI164566-01 PAVE/MDC; R01 NS107022 03 Measurement and Characterization of CNS and Immune Tissue Myeloid HIV-1 Reservoirs), Pfizer (C4671026/CT202203680), and UPitt Clinical and Translational Science Institute (CTSI; UL1TR001857 04); participation on the DSMB for the Tatelo Project; participation on external Advisory Boards for Boston University CTSI, University of Utah CTSI, Northern New England CTSI, and Duke University Center for AIDS Research; and positions on the Board of Directors for Management Sciences for Health and i2b2 tranSMART. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- Global Health 50/50. The Sex, Gender and COVID-19 Health Policy Portal. Available at: https://globalhealth5050.org/the-sex-gender-and-covid-19-project/policy-.... Accessed 15 June 2023.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

