Amplification of Wild-Type RET Represents a Novel Molecular Subtype of Several Cancer Types With Clinical Response to Selpercatinib
- PMID: 37972337
- PMCID: PMC10681403
- DOI: 10.1200/PO.23.00295
Amplification of Wild-Type RET Represents a Novel Molecular Subtype of Several Cancer Types With Clinical Response to Selpercatinib
Abstract
Purpose: RET rearrangements and RET activating point mutations represent targetable genomic alterations in advanced solid tumors. However, the frequency and clinicopathologic characteristics of wild-type RET amplification in cancer and its potential role as a targetable oncogenic driver are not well-characterized.
Methods: In two institutional cohorts of patients with solid cancers from the Dana-Farber Cancer Institute (DFCI) and Memorial Sloan Kettering Cancer Center (MSKCC) whose tumors underwent next-generation sequencing (NGS), the frequency and clinicopathologic features of wild-type RET amplification in the absence of RET rearrangements or activating mutations was assessed. The findings were validated using merged data from The Cancer Genome Atlas (TCGA), Genomics Evidence Neoplasia Information Exchange (GENIE), and China Pan-Cancer data sets.
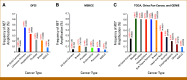
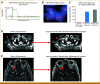
Results: The frequency of wild-type RET amplification across all solid cancers was 0.08% (26 of 32,505) in the DFCI cohort, 0.05% (26 of 53,152) in the MSKCC cohort, and 0.25% (71 of 28,623) in the cohort from TCGA, GENIE, and China Pan-Cancer. Cancer types with RET amplification included non-small-cell lung cancer (NSCLC), hepatobiliary cancer, prostate cancer, breast cancer, and others. The median RET copy number in RET-amplified cases was 7.5 (range, 6-36) in the DFCI cohort and 5.7 (range, 4-27.7) in the MSKCC cohort. Among 11 RET-amplified NSCLCs, eight had no other concurrent driver mutations. Finally, we report on a 69-year-old man with recurrent NSCLC harboring high-level wild-type RET amplification (22-28 copies) as the only identified putative genomic driver who experienced both a systemic and intracranial confirmed response to the RET inhibitor selpercatinib.
Conclusion: Amplification of wild-type RET represents a novel, targetable molecular subset of cancer.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
Similar articles
-
Mechanisms of resistance to selective RET tyrosine kinase inhibitors in RET fusion-positive non-small-cell lung cancer.Ann Oncol. 2020 Dec;31(12):1725-1733. doi: 10.1016/j.annonc.2020.09.015. Epub 2020 Sep 29. Ann Oncol. 2020. PMID: 33007380 Free PMC article.
-
Overcoming MET-Dependent Resistance to Selective RET Inhibition in Patients with RET Fusion-Positive Lung Cancer by Combining Selpercatinib with Crizotinib.Clin Cancer Res. 2021 Jan 1;27(1):34-42. doi: 10.1158/1078-0432.CCR-20-2278. Epub 2020 Oct 20. Clin Cancer Res. 2021. PMID: 33082208 Free PMC article.
-
Selpercatinib in RET-fusion positive metastatic non-small cell lung cancer: achievements and gray areas.Expert Rev Anticancer Ther. 2022 Aug;22(8):785-794. doi: 10.1080/14737140.2022.2093190. Epub 2022 Jun 27. Expert Rev Anticancer Ther. 2022. PMID: 35726802 Review.
-
RET rearrangements in non-small cell lung cancer: Evolving treatment landscape and future challenges.Biochim Biophys Acta Rev Cancer. 2022 Nov;1877(6):188810. doi: 10.1016/j.bbcan.2022.188810. Epub 2022 Oct 4. Biochim Biophys Acta Rev Cancer. 2022. PMID: 36202311 Review.
-
Therapeutic Advances in the Management of Patients with Advanced RET Fusion-Positive Non-Small Cell Lung Cancer.Curr Treat Options Oncol. 2021 Jun 24;22(8):72. doi: 10.1007/s11864-021-00867-8. Curr Treat Options Oncol. 2021. PMID: 34165651 Review.
Cited by
-
RET-Altered Cancers-A Tumor-Agnostic Review of Biology, Diagnosis and Targeted Therapy Activity.Cancers (Basel). 2023 Aug 17;15(16):4146. doi: 10.3390/cancers15164146. Cancers (Basel). 2023. PMID: 37627175 Free PMC article. Review.
-
Adverse event profiles of selpercatinib: a real-world pharmacovigilance analysis based on FAERS database.BMC Cancer. 2024 Dec 3;24(1):1486. doi: 10.1186/s12885-024-13250-1. BMC Cancer. 2024. PMID: 39627756 Free PMC article.
-
Effective treatment of non-fusion RET intragenic deletion lung adenocarcinoma with pralsetinib: a case report.Front Med (Lausanne). 2025 Jun 18;12:1546287. doi: 10.3389/fmed.2025.1546287. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40606448 Free PMC article.
References
-
- Shabbir A Kojadinovic A Shafiq T, et al. : Targeting RET alterations in cancer: Recent progress and future directions. Crit Rev Oncol Hematol 181:103882, 2023 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

