Clinical Validation of Human Papilloma Virus Circulating Tumor DNA for Early Detection of Residual Disease After Chemoradiation in Cervical Cancer
- PMID: 37972346
- PMCID: PMC10824379
- DOI: 10.1200/JCO.23.00954
Clinical Validation of Human Papilloma Virus Circulating Tumor DNA for Early Detection of Residual Disease After Chemoradiation in Cervical Cancer
Abstract
Purpose: Most cervical cancers are caused by human papilloma virus (HPV), and HPV circulating tumor DNA (ctDNA) may identify patients at highest risk of relapse. Our pilot study using digital polymerase chain reaction (dPCR) showed that detectable HPV ctDNA at the end of chemoradiation (CRT) is associated with inferior progression-free survival (PFS) and that a next-generation sequencing approach (HPV-seq) may outperform dPCR. We aimed to prospectively validate HPV ctDNA as a tool for early detection of residual disease.
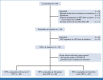
Methods: This prospective, multicenter validation study accrued patients with stage IB-IVA cervical cancer treated with CRT between 2017 and 2022. Participants underwent phlebotomy at baseline, end of CRT, 4-6 weeks post-CRT, and 3 months post-CRT for HPV ctDNA levels. Plasma HPV genotype-specific DNA levels were quantified using both dPCR and HPV-seq. The primary end point was 2-year PFS.
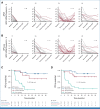
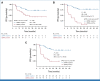
Results: With a median follow-up of 2.2 (range, 0.5-5.5) years, there were 24 PFS events among the 70 patients with HPV+ cervical cancer. Patients with detectable HPV ctDNA on dPCR at the end of CRT, 4-6 weeks post-CRT, and 3 months post-CRT had significantly worse 2-year PFS compared with those with undetectable HPV ctDNA (77% v 51%, P = .03; 82% v 15%, P < .001; and 82% v 24%, P < .001, respectively); the median lead time to recurrence was 5.9 months. HPV-seq showed similar results as dPCR. On multivariable analyses, detectable HPV ctDNA on dPCR and HPV-seq remained independently associated with inferior PFS.
Conclusion: Persistent HPV ctDNA after CRT is independently associated with inferior PFS. HPV ctDNA testing can identify, as early as at the end of CRT, patients at high risk of recurrence for future treatment intensification trials.
Trial registration: ClinicalTrials.gov NCT03853915 NCT03702309.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- Pötter R, Tanderup K, Schmid MP, et al. : MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): A multicentre prospective cohort study. Lancet Oncol 22:538-547, 2021 - PubMed
-
- Rose PG, Java J, Whitney CW, et al. : Nomograms predicting progression-free survival, overall survival, and pelvic recurrence in locally advanced cervical cancer developed from an analysis of identifiable prognostic factors in patients from NRG Oncology/Gynecologic Oncology Group randomized trials of chemoradiotherapy. J Clin Oncol 33:2136-2142, 2015 - PMC - PubMed
-
- Han K, Leung E, Barbera L, et al. : Circulating human papillomavirus DNA as a biomarker of response in patients with locally advanced cervical cancer treated with definitive chemoradiation. JCO Precis Oncol 2:1-8, 2018 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

