LIGHTSITE III: 13-Month Efficacy and Safety Evaluation of Multiwavelength Photobiomodulation in Nonexudative (Dry) Age-Related Macular Degeneration Using the Lumithera Valeda Light Delivery System
- PMID: 37972955
- PMCID: PMC10885856
- DOI: 10.1097/IAE.0000000000003980
LIGHTSITE III: 13-Month Efficacy and Safety Evaluation of Multiwavelength Photobiomodulation in Nonexudative (Dry) Age-Related Macular Degeneration Using the Lumithera Valeda Light Delivery System
Abstract
Purpose: The LIGHTSITE III study evaluated multiwavelength photobiomodulation (PBM) therapy in nonexudative (dry) age-related macular degeneration (AMD) using the LumiThera Valeda Light Delivery System.
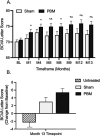
Methods: LIGHTSITE III is a randomized, controlled trial to assess the safety and effectiveness of PBM in dry AMD. Subjects were given multiwavelength PBM (590, 660, and 850 nm) or Sham treatment delivered in a series of nine sessions over 3 to 5 weeks every four months over 24 months. Subjects were assessed for efficacy and safety outcomes. Data from the 13-month analysis are presented in this report.
Results: A total of 100 subjects (148 eyes) with dry AMD were randomized. LIGHTSITE III met the primary efficacy best-corrected visual acuity endpoint with a significant difference between PBM (n = 91 eyes) and Sham (n = 54 eyes) groups (Between group difference: 2.4 letters (SE 1.15), CI: -4.7 to -0.1, P = 0.02) (PBM alone: 5.4 letters (SE 0.96), CI: 3.5 to 7.3, P < 0.0001; Sham alone: 3.0 letters (SE 1.13), CI: 0.7-5.2, P < 0.0001). The PBM group showed a significant decrease in new onset geographic atrophy ( P = 0.024, Fisher exact test, odds ratio 9.4). A favorable safety profile was observed.
Conclusion: LIGHTSITE III provides a prospective, randomized, controlled trial showing improved clinical and anatomical outcomes in intermediate dry AMD following PBM therapy.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the Opthalmic Communications Society, Inc.
Conflict of interest statement
S. E. Tedford, C. L. Croissant, M. Walker, R. Rückert and C. Tedford are all employees/contractors of LumiThera.
Figures






References
-
- Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106–e116. - PubMed
-
- Evans J. Antioxidant supplements to prevent or slow down the progression of AMD: a systematic review and meta-analysis. Eye (Lond) 2008;22:751–760. - PubMed

