HSPA8 Activates Wnt/β-Catenin Signaling to Facilitate BRAF V600E Colorectal Cancer Progression by CMA-Mediated CAV1 Degradation
- PMID: 37973552
- PMCID: PMC10797426
- DOI: 10.1002/advs.202306535
HSPA8 Activates Wnt/β-Catenin Signaling to Facilitate BRAF V600E Colorectal Cancer Progression by CMA-Mediated CAV1 Degradation
Abstract
BRAF V600E attracts wide attention in the treatment of colorectal cancer (CRC) as stratifying and predicting a refractory classification of CRC. Recent evidence indicates that Wnt/β-catenin signaling is broadly activated and participates in the refractoriness of BRAF V600E CRC, but the underlying molecular mechanism needs to be elucidated. Here, heat shock 70 kDa protein 8 (HSPA8), an essential regulator in chaperone-mediated autophagy (CMA), is identified as a potential therapeutic target for advanced BRAF V600E CRC. These results show that HSPA8 is transcriptionally upregulated in BRAF V600E CRC, which promotes CMA-dependent degradation of caveolin-1 (CAV1) to release β-catenin into the nucleus and thus activates the Wnt/β-catenin pathway, contributing to metastasis and progression of BRAF V600E CRC. Of note, HSPA8 directly interacts with the KIFSN motif on CAV1, the interaction can be enhanced by p38 MAPK-mediated CAV1 S168 phosphorylation. Furthermore, pharmacological targeting HSPA8 by VER155008 exhibits synergistic effects with BRAF inhibitors on CRC mouse models. In summary, these findings discover the important role of the HSPA8/CAV1/β-catenin axis in the development of refractory BRAF V600E CRC and highlight HSPA8 as a predictive biomarker and therapeutic target in clinical practice.
Keywords: BRAF V600E; Wnt/β-catenin; drug resistance; epithelial-mesenchymal transition; heat shock 70 kDa protein 8.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

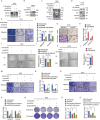

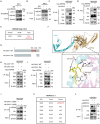
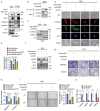

References
-
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., Bray F., CA Cancer J Clin 2021, 71, 209. - PubMed
-
- Siegel R. L., Miller K. D., Fuchs H. E., Jemal A., CA Cancer J Clin 2021, 71, 7. - PubMed
-
- Grothey A., Fakih M., Tabernero J., Ann. Oncol. 2021, 32, 959. - PubMed
-
- Prahallad A., Sun C., Huang S., Di Nicolantonio F., Salazar R., Zecchin D., Beijersbergen R. L., Bardelli A., Bernards R., Nature 2012, 483, 100. - PubMed
-
- a) Corcoran R. B., Ebi H., Turke A. B., Coffee E. M., Nishino M., Cogdill A. P., Brown R. D., Pelle P. D., Dias‐Santagata D., Hung K. E., Flaherty K. T., Piris A., Wargo J. A., Settleman J., Mino‐Kenudson M., Engelman J. A., Cancer Discovery 2012, 2, 227; - PMC - PubMed
- b) Yaeger R., Corcoran R. B., Cancer Discovery 2019, 9, 329. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2020YFA0509400/National Key Research and Development Project of China
- 2019B030302012/Guangdong Basic and Applied Basic Research Foundation
- 81821002/National Natural Science Foundation of China
- 82130082/National Natural Science Foundation of China
- 82341004/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
