Infant microbiome cultivation and metagenomic analysis reveal Bifidobacterium 2'-fucosyllactose utilization can be facilitated by coexisting species
- PMID: 37973815
- PMCID: PMC10654741
- DOI: 10.1038/s41467-023-43279-y
Infant microbiome cultivation and metagenomic analysis reveal Bifidobacterium 2'-fucosyllactose utilization can be facilitated by coexisting species
Abstract
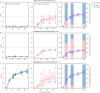
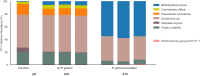
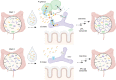
The early-life gut microbiome development has long-term health impacts and can be influenced by factors such as infant diet. Human milk oligosaccharides (HMOs), an essential component of breast milk that can only be metabolized by some beneficial gut microorganisms, ensure proper gut microbiome establishment and infant development. However, how HMOs are metabolized by gut microbiomes is not fully elucidated. Isolate studies have revealed the genetic basis for HMO metabolism, but they exclude the possibility of HMO assimilation via synergistic interactions involving multiple organisms. Here, we investigate microbiome responses to 2'-fucosyllactose (2'FL), a prevalent HMO and a common infant formula additive, by establishing individualized microbiomes using fecal samples from three infants as the inocula. Bifidobacterium breve, a prominent member of infant microbiomes, typically cannot metabolize 2'FL. Using metagenomic data, we predict that extracellular fucosidases encoded by co-existing members such as Ruminococcus gnavus initiate 2'FL breakdown, thus critical for B. breve's growth. Using both targeted co-cultures and by supplementation of R. gnavus into one microbiome, we show that R. gnavus can promote extensive growth of B. breve through the release of lactose from 2'FL. Overall, microbiome cultivation combined with genome-resolved metagenomics demonstrates that HMO utilization can vary with an individual's microbiome.
© 2023. The Author(s).
Conflict of interest statement
The Regents of the University of California have a patent pending related to this work on which Y.C.L., B.E.R., A.L.B., and J.F.B. are inventors. J.F.B. is a co-founder of Metagenomi. J.A.D. is a co-founder of Caribou Biosciences, Editas Medicine, Scribe Therapeutics, Intellia Therapeutics, and Mammoth Biosciences. J.A.D. is a scientific advisory board member of Vertex, Caribou Biosciences, Intellia Therapeutics, Scribe Therapeutics, Mammoth Biosciences, The Column Group, and Inari. J.A.D. is a Director at Johnson & Johnson, Altos Labs, and Tempus and has research projects sponsored by AppleTree Partners and Roche. B.E.R. is a shareholder of Caribou Biosciences, Intellia Therapeutics, Locus Biosciences, Inari, TreeCo, and Ancilia Biosciences. The authors declare no competing interests.
Figures