Host extracellular vesicles confer cytosolic access to systemic LPS licensing non-canonical inflammasome sensing and pyroptosis
- PMID: 37973841
- PMCID: PMC11111309
- DOI: 10.1038/s41556-023-01269-8
Host extracellular vesicles confer cytosolic access to systemic LPS licensing non-canonical inflammasome sensing and pyroptosis
Abstract
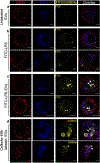
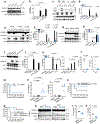
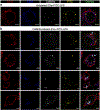
Intracellular surveillance for systemic microbial components during homeostasis and infections governs host physiology and immunity. However, a long-standing question is how circulating microbial ligands become accessible to intracellular receptors. Here we show a role for host-derived extracellular vesicles (EVs) in this process; human and murine plasma-derived and cell culture-derived EVs have an intrinsic capacity to bind bacterial lipopolysaccharide (LPS). Remarkably, circulating host EVs capture blood-borne LPS in vivo, and the LPS-laden EVs confer cytosolic access for LPS, triggering non-canonical inflammasome activation of gasdermin D and pyroptosis. Mechanistically, the interaction between the lipid bilayer of EVs and the lipid A of LPS underlies EV capture of LPS, and the intracellular transfer of LPS by EVs is mediated by CD14. Overall, this study demonstrates that EVs capture and escort systemic LPS to the cytosol licensing inflammasome responses, uncovering EVs as a previously unrecognized link between systemic microbial ligands and intracellular surveillance.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures





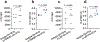







References
-
- Gabanyi I et al. Bacterial sensing via neuronal Nod2 regulates appetite and body temperature. Science 376, eabj3986 (2022). - PubMed
-
- Huang Z et al. Antibody neutralization of microbiota-derived circulating peptidoglycan dampens inflammation and ameliorates autoimmunity. Nat Microbiol 4, 766–773 (2019). - PubMed
-
- Moltke J von, Ayres, J. S., Kofoed, E. M., Chavarría-Smith, J. & Vance, R. E. Recognition of Bacteria by Inflammasomes. Annu Rev Immunol 31, 73–106 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

