BubbleDrive, a low-volume incubation chamber for acute brain slices
- PMID: 37973847
- PMCID: PMC10654715
- DOI: 10.1038/s41598-023-45949-9
BubbleDrive, a low-volume incubation chamber for acute brain slices
Erratum in
-
Author Correction: BubbleDrive, a low-volume incubation chamber for acute brain slices.Sci Rep. 2024 Jan 23;14(1):1981. doi: 10.1038/s41598-024-52441-5. Sci Rep. 2024. PMID: 38263205 Free PMC article. No abstract available.
Abstract
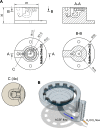
Acute brain slices are a common and useful preparation in experimental neuroscience. A wide range of incubation chambers for brain slices exists but only a few are designed with very low volumes of the bath solution in mind. Such chambers are necessary when high-cost chemicals are to be added to the solution or when small amounts of substances released by the slice are to be collected for analysis. The principal challenge in designing a very low-volume incubation chamber is maintaining good oxygenation and flow without mechanically disturbing or damaging the slices. We designed and validated BubbleDrive, a 3D-printed incubation chamber with a minimum volume of 1.5 mL which can hold up to three coronal mouse slices from one hemisphere. It employs the carbogen gas bubbles to drive the flow circulation in a consistent and reproducible manner, and without disturbing the brain slices. The BubbleDrive design and construction were successfully validated by comparison to a conventional large-volume incubation chamber in several experimental designs involving measurements of extracellular diffusion parameters, the electrophysiology of neuronal and astrocytic networks, and the effectiveness of slice incubation with hyaluronidase enzyme.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
Easy to build cost-effective acute brain slice incubation system for parallel analysis of multiple treatment conditions.J Neurosci Methods. 2022 Jan 15;366:109405. doi: 10.1016/j.jneumeth.2021.109405. Epub 2021 Nov 14. J Neurosci Methods. 2022. PMID: 34785269
-
A recording chamber for small volume slice electrophysiology.J Neurophysiol. 2015 Sep;114(3):2053-64. doi: 10.1152/jn.00289.2014. Epub 2015 Jul 22. J Neurophysiol. 2015. PMID: 26203105 Free PMC article.
-
Author Correction: BubbleDrive, a low-volume incubation chamber for acute brain slices.Sci Rep. 2024 Jan 23;14(1):1981. doi: 10.1038/s41598-024-52441-5. Sci Rep. 2024. PMID: 38263205 Free PMC article. No abstract available.
-
Brain slice on a chip: opportunities and challenges of applying microfluidic technology to intact tissues.Lab Chip. 2012 Jun 21;12(12):2103-17. doi: 10.1039/c2lc21142d. Epub 2012 Apr 25. Lab Chip. 2012. PMID: 22534786 Review.
-
Roles of astrocytic Na(+),K(+)-ATPase and glycogenolysis for K(+) homeostasis in mammalian brain.J Neurosci Res. 2015 Jul;93(7):1019-30. doi: 10.1002/jnr.23499. Epub 2014 Oct 29. J Neurosci Res. 2015. PMID: 25352321 Review.
Cited by
-
Translational insights into the hormetic potential of carbon dioxide: from physiological mechanisms to innovative adjunct therapeutic potential for cancer.Front Physiol. 2024 Jul 17;15:1415037. doi: 10.3389/fphys.2024.1415037. eCollection 2024. Front Physiol. 2024. PMID: 39086932 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

