Promising impact of push-pull configuration into designed octacyclic naphthalene-based organic scaffolds for nonlinear optical amplitudes: a quantum chemical approach
- PMID: 37973880
- PMCID: PMC10654730
- DOI: 10.1038/s41598-023-44327-9
Promising impact of push-pull configuration into designed octacyclic naphthalene-based organic scaffolds for nonlinear optical amplitudes: a quantum chemical approach
Abstract
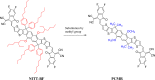
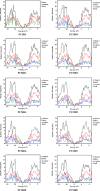
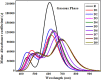
In opto-electronics, non-fullerene (NF) derivatives are regarded as efficient non-linear optical (NLO) materials. The present investigation was based on designing NF naphthalene-based derivatives (PCMD1-D9) with D-π-A configuration from PCMR. DFT analysis at M06/6-311G (d,p) level was accomplished to explore the photonic behavior of PCMD1-D9 compounds. Various kind of analysis like; UV-Vis, density of state (DOS), natural bond orbitals (NBOs), transition density matrix (TDM) and frontier molecular orbitals (FMOs) analyses were accomplished to understand the NLO properties of said chromophores. The configuration change led to considerable charge distribution over highest occupied and lowest unoccupied molecular orbitals with minimum band difference. The energy gap trend for all the entitled compounds was observed as; PCMD8 < PCMD5 = PCMD9 < PCMD6 < PCMD7 < PCMD4 < PCMD3 < PCMD2 < PCMD1 with the least band gap of 2.048 eV in PCMD8 among all the compounds. The UV-Visible spectrum of the entitled chromophores manifested high values of λmax in derivatives contrary to PCMR. Additionally, NBO findings explored effective intramolecular charge transfer and maximum energy of stabilization (34.31 kcal/mol) for PCMD8 chromophore. The highest linear polarizability (<α>) and dipole moment (µtot) values were exhibited by PCMD5 at 2.712 × 10-22. and 1.995 × 10-17 esu, respectively. PCMD8 push-pull configured molecular entity exhibited highest first hyper-polarizability (βtot) at 4.747 × 10-27 esu and second hyper-polarizability at 6.867 × 10-32 esu. Overall, all the formulated chromophores exhibited significant NLO results contrary to PCMR. Hence, through this structural tailoring via various acceptors, effective NLO materials were obtained for optoelectronic applications.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Brown F, Parks RE, Sleeper AM. Nonlinear optical reflection from a metallic boundary. Phys. Rev. Lett. 1965;14:1029. doi: 10.1103/PhysRevLett.14.1029. - DOI
-
- Castro MCR, Belsley M, Raposo MMM. Push–pull second harmonic generation chromophores bearing pyrrole and thiazole heterocycles functionalized with several acceptor moieties: Syntheses and characterization. Dyes Pigm. 2016;128:89–95. doi: 10.1016/j.dyepig.2016.01.015. - DOI
-
- Anis M, Shaikh RN, Shirsat MD, Hussaini SS. Investigation of optical and electrical properties of L-cystein doped zinc thiourea chloride (ZTC) crystal for nonlinear optical (NLO) applications. Opt. Laser Technol. 2014;60:124–129. doi: 10.1016/j.optlastec.2014.01.011. - DOI
-
- Bano R, et al. Diamondoid as potential nonlinear optical material by superalkali doping: A first principles study. Diam. Relat. Mater. 2023;135:109826. doi: 10.1016/j.diamond.2023.109826. - DOI
-
- Mahmood A, Abdullah MI, Nazar MF. Quantum chemical designing of novel organic non-linear optical compounds. Bull. Korean Chem. Soc. 2014;35:1391–1396. doi: 10.5012/bkcs.2014.35.5.1391. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

