Maternal vitamin B1 is a determinant for the fate of primordial follicle formation in offspring
- PMID: 37973927
- PMCID: PMC10654754
- DOI: 10.1038/s41467-023-43261-8
Maternal vitamin B1 is a determinant for the fate of primordial follicle formation in offspring
Abstract
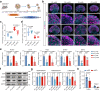
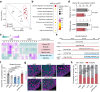
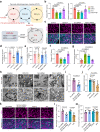
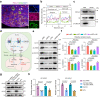
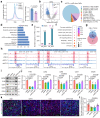
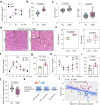
The mediation of maternal-embryonic cross-talk via nutrition and metabolism impacts greatly on offspring health. However, the underlying key interfaces remain elusive. Here, we determined that maternal high-fat diet during pregnancy in mice impaired preservation of the ovarian primordial follicle pool in female offspring, which was concomitant with mitochondrial dysfunction of germ cells. Furthermore, this occurred through a reduction in maternal gut microbiota-related vitamin B1 while the defects were restored via vitamin B1 supplementation. Intriguingly, vitamin B1 promoted acetyl-CoA metabolism in offspring ovaries, contributing to histone acetylation and chromatin accessibility at the promoters of cell cycle-related genes, enhancement of mitochondrial function, and improvement of granulosa cell proliferation. In humans, vitamin B1 is downregulated in the serum of women with gestational diabetes mellitus. In this work, these findings uncover the role of the non-gamete transmission of maternal high-fat diet in influencing offspring oogenic fate. Vitamin B1 could be a promising therapeutic approach for protecting offspring health.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

