Primate-specific ZNF808 is essential for pancreatic development in humans
- PMID: 37973953
- PMCID: PMC10703691
- DOI: 10.1038/s41588-023-01565-x
Primate-specific ZNF808 is essential for pancreatic development in humans
Abstract
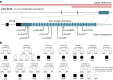
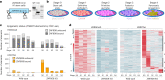
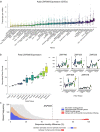
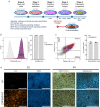
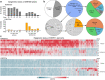
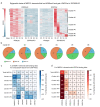
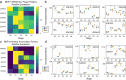
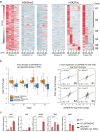
Identifying genes linked to extreme phenotypes in humans has the potential to highlight biological processes not shared with all other mammals. Here, we report the identification of homozygous loss-of-function variants in the primate-specific gene ZNF808 as a cause of pancreatic agenesis. ZNF808 is a member of the KRAB zinc finger protein family, a large and rapidly evolving group of epigenetic silencers which target transposable elements. We show that loss of ZNF808 in vitro results in aberrant activation of regulatory potential contained in the primate-specific transposable elements it represses during early pancreas development. This leads to inappropriate specification of cell fate with induction of genes associated with liver identity. Our results highlight the essential role of ZNF808 in pancreatic development in humans and the contribution of primate-specific regions of the human genome to congenital developmental disease.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

