Long-term humoral and cellular immunity after primary SARS-CoV-2 infection: a 20-month longitudinal study
- PMID: 37974069
- PMCID: PMC10652616
- DOI: 10.1186/s12865-023-00583-y
Long-term humoral and cellular immunity after primary SARS-CoV-2 infection: a 20-month longitudinal study
Abstract
Background: SARS-CoV-2 remains a world-wide health issue. SARS-CoV-2-specific immunity is induced upon both infection and vaccination. However, defining the long-term immune trajectory, especially after infection, is limited. In this study, we aimed to further the understanding of long-term SARS-CoV-2-specific immune response after infection.
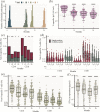
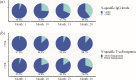
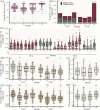
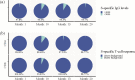
Results: We conducted a longitudinal cohort study among 93 SARS-CoV-2 recovered individuals. Immune responses were continuously monitored for up to 20 months after infection. The humoral responses were quantified by Spike- and Nucleocapsid-specific IgG levels. T cell responses to Spike- and non-Spike epitopes were examined using both intercellular cytokine staining (ICS) assay and Activation-Induced marker (AIM) assay with quantification of antigen-specific IFNγ production. During the 20 months follow-up period, Nucleocapsid-specific antibody levels and non-Spike-specific CD4 + and CD8 + T cell frequencies decreased in the blood. However, a majority of participants maintained a durable immune responses 20 months after infection: 59% of the participants were seropositive for Nucleocapsid-specific IgG, and more than 70% had persisting non-Spike-specific T cells. The Spike-specific response initially decreased but as participants were vaccinated against COVID-19, Spike-specific IgG levels and T cell frequencies were boosted reaching similar or higher levels compared to 1 month post-infection. The trajectory of infection-induced SARS-CoV-2-specific immunity decreases, but for the majority of participants it persists beyond 20 months. The T cell response displays a greater durability. Vaccination boosts Spike-specific immune responses to similar or higher levels as seen after primary infection.
Conclusions: For most participants, the response persists 20 months after infection, and the cellular response appears to be more long-lived compared to the circulating antibody levels. Vaccination boosts the S-specific response but does not affect the non-S-specific response. Together, these findings support the understanding of immune contraction, and with studies showing the immune levels required for protection, adds to the knowledge of durability of protection against future SARS-CoV-2.
Keywords: Antibodies; Antigen-specific T cells; Immune durability; Infection; SARS-CoV-2; Vaccine.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Statement on the fifteenth meeting of the IHR. Emergency Committee on the COVID-19 pandemic; 2005. https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meet.... Accessed 15 June 2023.
-
- Staerke NB, Reekie J, Nielsen H, Benfield T, Wiese L, Knudsen LS, Iversen MB, Iversen K, Fogh K, Bodilsen J, et al. Levels of SARS-CoV-2 antibodies among fully vaccinated individuals with Delta or Omicron variant breakthrough infections. Nat Commun. 2022;13(1):4466. doi: 10.1038/s41467-022-32254-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

