Study on maintenance of eyeball morphology by foldable capsular vitreous body in severe ocular trauma
- PMID: 37974090
- PMCID: PMC10655399
- DOI: 10.1186/s12886-023-03209-4
Study on maintenance of eyeball morphology by foldable capsular vitreous body in severe ocular trauma
Abstract
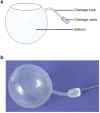
Objectives: To explore the feasibility and safety of using a foldable capsular vitreous body (FCVB) in managing severe ocular trauma and silicone oil-dependent eyes.
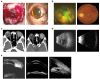
Methodology: This is a retrospective study of 61 ocular trauma patients (61 eyes) who presented to the Department of Eye Emergency, Hebei Eye Hospital from May 1, 2018, to May 31, 2019, including 51 male patients (51 eyes) and 10 female patients (10 eyes) with an average age of 44.98 ± 14.60 years old. The oldest patient was 75 years old, and the youngest was 8 years old. These cases represented 51 eyes with severe eyeball rupture and 10 eyes with severe, complicated ocular trauma, which became silicone oil-dependent after the operation. These patients received FCVB implants, and data regarding their visual acuity, intraocular pressure, changes in eye axis, cornea, retina, and FCVB state were recorded after the operation.
Results: In all patients, the FCVB was properly positioned and well supported with the retina. All 61 patients cleared a follow-up window of 1-36 months with no reports of important changes in their visual acuity. Among the patients, 91.8% reported normal intraocular pressure, the retinal reattachment rate reached 100%, and the eyeball atrophy control rate reached 100%. There was no report of rupture of the FCVB, allergies to silicone, intraocular infection, intraocular hemorrhage, silicone oil emulsification, or sympathetic ophthalmia.
Conclusions: Foldable capsular vitreous bodies (FCVBs) designed to mimic natural vitreous bodies are suitable as long-term ocular implants that can provide sustained support for the retina without the need for any special postoperative postures. Their barrier function may effectively prolong the retention time of the tamponade and prevent various complications caused by direct contact of the eye tissues with the tamponade.
Keywords: Foldable capsular vitreous body; Intraocular pressure; Ocular trauma; Retina; Severe case; Visual acuity.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Study on the effectiveness and safety of Foldable Capsular Vitreous Body implantation.BMC Ophthalmol. 2019 Dec 18;19(1):260. doi: 10.1186/s12886-019-1268-x. BMC Ophthalmol. 2019. PMID: 31852464 Free PMC article.
-
Reattachment After Foldable Capsular Vitreous Body Implantation in Severe Retinal Detachment Eyes.Transl Vis Sci Technol. 2021 Sep 1;10(11):8. doi: 10.1167/tvst.10.11.8. Transl Vis Sci Technol. 2021. PMID: 34491287 Free PMC article.
-
Preliminary clinical application of foldable capsular vitreous body in severe silicone oil-dependent eyes.Ann Palliat Med. 2021 Oct;10(10):10922-10929. doi: 10.21037/apm-21-2554. Ann Palliat Med. 2021. PMID: 34763454
-
Foldable capsular vitreous body indications, complications, and outcomes: a systematic review.Graefes Arch Clin Exp Ophthalmol. 2023 Aug;261(8):2103-2116. doi: 10.1007/s00417-023-05995-5. Epub 2023 Feb 16. Graefes Arch Clin Exp Ophthalmol. 2023. PMID: 36795160
-
Desired properties of polymeric hydrogel vitreous substitute.Biomed Pharmacother. 2024 Mar;172:116154. doi: 10.1016/j.biopha.2024.116154. Epub 2024 Feb 1. Biomed Pharmacother. 2024. PMID: 38306844 Review.
Cited by
-
Consensus on the Treatment of Severe Ocular Trauma and Silicone Oil-Dependent Eyes Using Foldable Capsular Vitreous Body.J Evid Based Med. 2025 Jun;18(2):e70041. doi: 10.1111/jebm.70041. J Evid Based Med. 2025. PMID: 40571677 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

