Iron and copper: critical executioners of ferroptosis, cuproptosis and other forms of cell death
- PMID: 37974196
- PMCID: PMC10652626
- DOI: 10.1186/s12964-023-01267-1
Iron and copper: critical executioners of ferroptosis, cuproptosis and other forms of cell death
Abstract
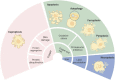
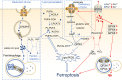
Regulated cell death (RCD) is a regulable cell death that involves well-organized signaling cascades and molecular mechanisms. RCD is implicated in fundamental processes such as organ production and tissue remodeling, removing superfluous structures or cells, and regulating cell numbers. Previous studies have not been able to reveal the complete mechanisms, and novel methods of RCD are constantly being proposed. Two metal ions, iron (Fe) and copper (Cu) are essential factors leading to RCDs that not only induce ferroptosis and cuproptosis, respectively but also lead to cell impairment and eventually diverse cell death. This review summarizes the direct and indirect mechanisms by which Fe and Cu impede cell growth and the various forms of RCD mediated by these two metals. Moreover, we aimed to delineate the interrelationships between these RCDs with the distinct pathways of ferroptosis and cuproptosis, shedding light on the complex and intricate mechanisms that govern cellular survival and death. Finally, the prospects outlined in this review suggest a novel approach for investigating cell death, which may involve integrating current therapeutic strategies and offer a promising solution to overcome drug resistance in certain diseases. Video Abstract.
Keywords: Copper; Cuproptosis; Ferroptosis; Iron; Proteasome inhibition; RCD; ROS.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

