Contribution of macrophages to neural survival and intracochlear tissue remodeling responses following cochlear implantation
- PMID: 37974203
- PMCID: PMC10652501
- DOI: 10.1186/s12974-023-02955-y
Contribution of macrophages to neural survival and intracochlear tissue remodeling responses following cochlear implantation
Abstract
Background: Cochlear implants (CIs) restore hearing to deafened patients. The foreign body response (FBR) following cochlear implantation (post-CI) comprises an infiltration of macrophages, other immune and non-immune cells, and fibrosis into the scala tympani, a space that is normally devoid of cells. This FBR is associated with negative effects on CI outcomes including increased electrode impedances and loss of residual acoustic hearing. This study investigates the extent to which macrophage depletion by an orally administered CSF-1R specific kinase (c-FMS) inhibitor, PLX-5622, modulates the tissue response to CI and neural health.
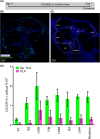
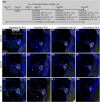
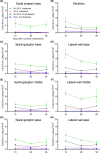
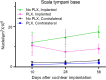
Main text: 10- to 12-week-old CX3CR1 + /GFP Thy1 + /YFP mice on C57BL/6J/B6 background was fed chow containing 1200 mg/kg PLX5622 or control chow for the duration of the study. 7 days after starting the diet, 3-channel cochlear implants were implanted in the ear via the round window. Serial impedance and neural response telemetry (NRT) measurements were acquired throughout the study. Electric stimulation began 7 days post-CI until 28 days post-CI for 5 h/day, 5 days/week, with programming guided by NRT and behavioral responses. Cochleae harvested at 10, 28 or 56 days post-CI were cryosectioned and labeled with an antibody against α-smooth muscle actin (α-SMA) to identify myofibroblasts and quantify the fibrotic response. Using IMARIS image analysis software, the outlines of scala tympani, Rosenthal canal, modiolus, and lateral wall for each turn were traced manually to measure region volume. The density of nuclei, CX3CR1 + macrophages, Thy1 + spiral ganglion neuron (SGN) numbers, and the ratio of the α-SMA + volume/scala tympani volume were calculated. Cochlear implantation in control diet subjects caused infiltration of cells, including macrophages, into the cochlea. Fibrosis was evident in the scala tympani adjacent to the electrode array. Mice fed PLX5622 chow showed reduced macrophage infiltration throughout the implanted cochleae across all time points. However, scala tympani fibrosis was not reduced relative to control diet subjects. Further, mice treated with PLX5622 showed increased electrode impedances compared to controls. Finally, treatment with PLX5622 decreased SGN survival in implanted and contralateral cochleae.
Conclusion: The data suggest that macrophages play an important role in modulating the intracochlear tissue response following CI and neural survival.
Keywords: Biomaterials; Cochlear implant; Fibrosis; Foreign body response; Inflammation.
© 2023. The Author(s).
Conflict of interest statement
Marlan R. Hansen is a co-founder and Chief Medical Officer of iotaMotion Inc. and a co-founder of ZwiCoat Materials Innovations, Inc with equity interest.
Figures



Update of
-
Contribution of macrophages to intracochlear tissue remodeling responses following cochlear implantation and neural survival.Res Sq [Preprint]. 2023 Jun 26:rs.3.rs-3065630. doi: 10.21203/rs.3.rs-3065630/v1. Res Sq. 2023. Update in: J Neuroinflammation. 2023 Nov 16;20(1):266. doi: 10.1186/s12974-023-02955-y. PMID: 37461619 Free PMC article. Updated. Preprint.
References
-
- Ahmadi N, Gausterer JC, Honeder C, Motz M, Schopper H, Zhu C, et al. Long-term effects and potential limits of intratympanic dexamethasone-loaded hydrogels combined with dexamethasone-eluting cochlear electrodes in a low-insertion trauma Guinea pig model. Hear Res. 2019;384:107825. doi: 10.1016/j.heares.2019.107825. - DOI - PubMed
-
- Bas E, Bohorquez J, Goncalves S, Perez E, Dinh CT, Garnham C, Van De Water TR, et al. Electrode array-eluted dexamethasone protects against electrode insertion trauma induced hearing and hair cell losses, damage to neural elements, increases in impedance and fibrosis: a dose response study. Hear Res. 2016;337:12–24. doi: 10.1016/j.heares.2016.02.003. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

