CCL5 mediated astrocyte-T cell interaction disrupts blood-brain barrier in mice after hemorrhagic stroke
- PMID: 37974301
- PMCID: PMC10870968
- DOI: 10.1177/0271678X231214838
CCL5 mediated astrocyte-T cell interaction disrupts blood-brain barrier in mice after hemorrhagic stroke
Abstract
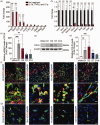
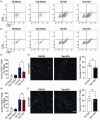
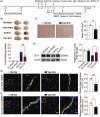
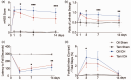
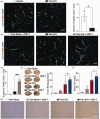
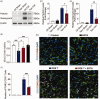
The crosstalk between reactive astrocytes and infiltrated immune cells plays a critical role in maintaining blood-brain barrier (BBB) integrity. However, how astrocytes interact with immune cells and the effect of their interaction on BBB integrity after hemorrhagic stroke are still unclear. By performing RNA sequencing in astrocytes that were activated by interleukin-1α (IL-1α), tumor necrosis factor α (TNFα), and complement component 1q (C1q) treatment, we found CCL5 was among the top upregulated genes. Immunostaining and western blot results demonstrated that CCL5 was increased in mice brain after hemorrhagic stroke. Flow cytometry showed that knockout of astrocytic CCL5 reduced the infiltration of CD8+ but not CD4+ T and myeloid cells into the brain (p < 0.05). In addition, knockout CCL5 in astrocytes increased tight junction-related proteins ZO-1 and Occludin expression; reduced Evans blue leakage, perforin and granzyme B expression; improved neurobehavioral outcomes in hemorrhagic stroke mice (p < 0.05), while transplantation of CD8+ T cells reversed these protective effects. Moreover, co-culture of CD8+ T cells with bEnd.3 cells induced the apoptosis of bEnd.3 cells, which was rescued by inhibiting perforin. In conclusion, our study suggests that CCL5 mediated crosstalk between astrocytes and CD8+ T cells represents an important therapeutic target for protecting BBB in stroke.
Keywords: Astrocytes; CCL5; T cells; blood-brain barrier; intracerebral hemorrhage.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Pivoriūnas A, Verkhratsky A. Astrocyte–endotheliocyte axis in the regulation of the blood–brain barrier. Neurochem Res 2021; 46: 2538–2550. - PubMed
-
- Sukumari-Ramesh S, Alleyne CH, Dhandapani KM. Astrogliosis: a target for intervention in intracerebral hemorrhage? Transl Stroke Res 2012; 3: 80–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

