A cardiotoxicity-eliminated ACE2 variant as a pan-inhibitor against coronavirus cell invasion
- PMID: 37974399
- PMCID: PMC10787150
- DOI: 10.1016/j.ymthe.2023.11.019
A cardiotoxicity-eliminated ACE2 variant as a pan-inhibitor against coronavirus cell invasion
Abstract
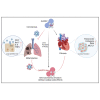
Human recombinant ACE2 (hrACE2) has been highly anticipated as a successful COVID-19 treatment; however, its potential to cause cardiac side effects has given rise to many concerns. Here, we developed a cardiotoxicity-eliminated hrACE2 variant, which had four mutation sites within hrACE2 (H345L, H374L, H378L, H505L) and was named as hrACE2-4mu. hrACE2-4mu has a consistent binding affinity with the variant SARS-CoV-2 spike proteins (SPs) and an efficient ability to block SP-induced SARS-CoV-2 entry into cells. In golden hamsters, injection of purified wild-type (WT) hrACE2 rescues the early stages of pneumonia caused by the SPs of the WT, delta, and omicron variants with reduced inflammatory cell infiltration. However, long-term injection of WT hrACE2 induces undesired cardiac fibrosis, as demonstrated by upregulated fibronectin and collagen expression. Our newly developed hrACE2-4mu showed similar protective abilities against a series of coronavirus cell invasions as WT hrACE2, meanwhile it did not cause apparent cardiac side effects. Thus, we generated a cardiotoxicity-eliminated variant of hrACE2 as a pan-inhibitor against coronavirus cell invasion, providing a potential novel strategy for the treatment of COVID-19 and other coronaviruses.
Keywords: ACE2; cardiac fibrosis; coronavirus; pan-inhibitor; spike protein.
Copyright © 2023 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
-
- Haschke M., Schuster M., Poglitsch M., Loibner H., Salzberg M., Bruggisser M., Penninger J., Krähenbühl S. Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clin. Pharmacokinet. 2013;52:783–792. - PubMed
-
- Masson R., Nicklin S.A., Craig M.A., McBride M., Gilday K., Gregorevic P., Allen J.M., Chamberlain J.S., Smith G., Graham D., et al. Onset of experimental severe cardiac fibrosis is mediated by overexpression of Angiotensin-converting enzyme 2. Hypertension. 2009;53:694–700. - PubMed
-
- Donoghue M., Wakimoto H., Maguire C.T., Acton S., Hales P., Stagliano N., Fairchild-Huntress V., Xu J., Lorenz J.N., Kadambi V., et al. Heart block, ventricular tachycardia, and sudden death in ACE2 transgenic mice with downregulated connexins. J. Mol. Cell. Cardiol. 2003;35:1043–1053. - PubMed
-
- Dodge S.M., Beardslee M.A., Darrow B.J., Green K.G., Beyer E.C., Saffitz J.E. Effects of angiotensin II on expression of the gap junction channel protein connexin43 in neonatal rat ventricular myocytes. J. Am. Coll. Cardiol. 1998;32:800–807. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

