AAV-mediated hepatic LPL expression ameliorates severe hypertriglyceridemia and acute pancreatitis in Gpihbp1 deficient mice and rats
- PMID: 37974401
- PMCID: PMC10787151
- DOI: 10.1016/j.ymthe.2023.11.018
AAV-mediated hepatic LPL expression ameliorates severe hypertriglyceridemia and acute pancreatitis in Gpihbp1 deficient mice and rats
Abstract
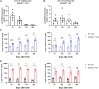
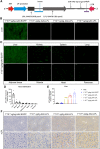
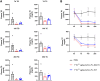
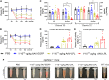
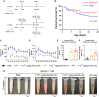
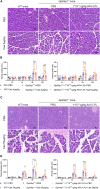
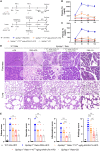
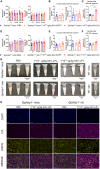
GPIHBP1 plays an important role in the hydrolysis of triglyceride (TG) lipoproteins by lipoprotein lipases (LPLs). However, Gpihbp1 knockout mice did not develop hypertriglyceridemia (HTG) during the suckling period but developed severe HTG after weaning on a chow diet. It has been postulated that LPL expression in the liver of suckling mice may be involved. To determine whether hepatic LPL expression could correct severe HTG in Gpihbp1 deficiency, liver-targeted LPL expression was achieved via intravenous administration of the adeno-associated virus (AAV)-human LPL gene, and the effects of AAV-LPL on HTG and HTG-related acute pancreatitis (HTG-AP) were observed. Suckling Gpihbp1-/- mice with high hepatic LPL expression did not develop HTG, whereas Gpihbp1-/- rat pups without hepatic LPL expression developed severe HTG. AAV-mediated liver-targeted LPL expression dose-dependently decreased plasma TG levels in Gpihbp1-/- mice and rats, increased post-heparin plasma LPL mass and activity, decreased mortality in Gpihbp1-/- rat pups, and reduced the susceptibility and severity of both Gpihbp1-/- animals to HTG-AP. However, the muscle expression of AAV-LPL had no significant effect on HTG. Targeted expression of LPL in the liver showed no obvious adverse reactions. Thus, liver-targeted LPL expression may be a new therapeutic approach for HTG-AP caused by GPIHBP1 deficiency.
Keywords: GPIHBP1; acute pancreatitis; adeno-associated virus; gene therapy; hypertriglyceridemia; lipoprotein lipase.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
References
-
- Simha V. Management of hypertriglyceridemia. BMJ. 2020;371:m3109. - PubMed
-
- Pirillo A., Casula M., Olmastroni E., Norata G.D., Catapano A.L. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 2021;18:689–700. - PubMed
-
- Cai S.J., Wong D.M., Chen S.H., Chan L. Structure of the human hepatic triglyceride lipase gene. Biochemistry. 1989;28:8966–8971. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

