Microwave-Driven Exsolution of Ni Nanoparticles in A-Site Deficient Perovskites
- PMID: 37974412
- PMCID: PMC10722607
- DOI: 10.1021/acsnano.3c08534
Microwave-Driven Exsolution of Ni Nanoparticles in A-Site Deficient Perovskites
Abstract
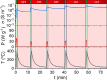
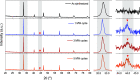
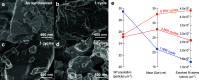
Exsolution has emerged as a promising method for generating metallic nanoparticles, whose robustness and stability outperform those of more conventional deposition methods, such as impregnation. In general, exsolution involves the migration of transition metal cations, typically perovskites, under reducing conditions, leading to the nucleation of well-anchored metallic nanoparticles on the oxide surface with particular properties. There is growing interest in exploring alternative methods for exsolution that do not rely on high-temperature reduction via hydrogen. For example, utilizing electrochemical potentials or plasma technologies has shown promising results in terms of faster exsolution, leading to better dispersion of nanoparticles under milder conditions. To avoid limitations in scaling up exhibited by electrochemical cells and plasma-generation devices, we proposed a method based on pulsed microwave (MW) radiation to drive the exsolution of metallic nanoparticles. Here, we demonstrate the H2-free MW-driven exsolution of Ni nanoparticles from lanthanum strontium titanates, characterizing the mechanism that provides control over nanoparticle size and dispersion and enhanced catalytic activity and stability for CO2 hydrogenation. The presented method will enable the production of metallic nanoparticles with a high potential for scalability, requiring short exposure times and low temperatures.
Keywords: exsolution; hydrogenation; microwave; nanoparticle nucleation; nickel; perovskite.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Zhang J.; Gao M. M.-R.; Luo J.-L. In Situ Exsolved Metal Nanoparticles: A Smart Approach for Optimization of Catalysts. Chem. Mater. 2020, 32 (13), 5424–5441. 10.1021/acs.chemmater.0c00721. - DOI
-
- Bhalla A. S.; Guo R.; Roy R. The Perovskite Structure—a Review of Its Role in Ceramic Science and Technology. Materials Research Innovations 2000, 4 (1), 3–26. 10.1007/s100190000062. - DOI
LinkOut - more resources
Full Text Sources

