Long-Term Control of Retinal Thickness Variability and Vision Following the 0.19 mg Fluocinolone Acetonide Implant
- PMID: 37974917
- PMCID: PMC10649457
- DOI: 10.1177/24741264231201314
Long-Term Control of Retinal Thickness Variability and Vision Following the 0.19 mg Fluocinolone Acetonide Implant
Abstract
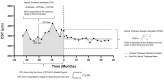
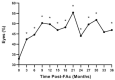
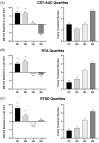
Purpose: To assess the impact of retinal thickness variability (RTV) control on visual and treatment burden outcomes in patients with diabetic macular edema (DME) who received the 0.19 mg fluocinolone acetonide (FAc) intravitreal implant (Iluvien, Alimera Sciences). Methods: This post hoc analysis examined the outcomes of a 3-year, phase 4, nonrandomized, open-label observational study. Retinal thickness was measured as central subfield thickness (CST). RTV was quantified by CST area under the curve (CST-AUC), retinal thickness amplitude (RTA), and retinal thickness standard deviation (RTSD). Visual outcomes were measured as best-corrected visual acuity (BCVA), and treatment burden was measured as the number of yearly supplemental DME treatments. Results: The percentage of eyes with a CST ≤300 µm fluctuated throughout the study but was significantly increased relative to baseline at 36 months (baseline: 32.9% vs 36 months: 46.8%; P < .05). FAc significantly reduced RTV in all measures more than 36 months (P < .0001). When divided into quartiles, eyes with the best RTV control post FAc had the greatest BCVA gains and improved disease control (ie, reduced need for supplemental therapy). The last-observed BCVA letter score exhibited linear correlations with CST-AUC (R2 = -0.100), RTA (R2 = -0.125), and RTSD (R2 = -0.162). A multivariate linear regression with baseline BCVA as a covariate displayed improved correlations with the last-observed BCVA, CST-AUC (R2 = -0.448), RTA (R2 = -0.432), and RTSD (R2 = -0.436). Conclusions: The sustained corticosteroid release of the 0.19 mg FAc implant reduced RTV in patients with DME, which directly correlated with significantly improved vision and a reduced supplemental treatment burden.
Keywords: diabetic macular edema; fluocinolone acetonide; intravitreal implant; retinal thickness; treatment burden.
© The Author(s) 2023.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Sheth received financial support from 4D Molecular Therapeutics, Allergan, Ashvattha Therapeutics, Chengdu Kanghong, Eyepoint, Genentech, Gyroscope, Ionis, Iveric Bio, NGM Biopharmaceuticals, Novartis, Opthea, Outlook, Oxurion, Recens Medical, Regeneron, Regenxbio, Roche, SalutarisMD, and SamChungDang, and Santen; is a consultant to Alimera Sciences, Apellis, Eyepoint, Genentech, Graybug, Iveric Bio, Novartis, Regeneron, and Vial; and is a lecturer for Alimera Sciences, Apellis, and Genentech. Dr. Singer is a consultant to Aerie, Alimera Sciences, Allegro, Allergan Eyepoint, Genentech Kodiak, Novartis, Regeneron, and Santen; is a lecturer for Aerie, Alimera, Sciences, Allegro, DRCR, Genentech, Icon, Ionis, Kalvista, Kodiak, Novartis, Opthea, Optos, Regeneron, Santen, Senju, and Sydnexis; provides expert testimony for Allergan, Genentech, Mallinckrodt, Regeneron, and Spark; and owns equity in Aviceda, Inflammasome, Novartis, and Nanoscope. Dr. MacCumber is a consultant to Alimera Sciences, Bausch + Lomb, Genentech, Novartis, and Regeneron. Dr. Cutino is an employee of Alimera Sciences and owns equity in Alimera Sciences. Dr. Kasper is an employee of Alimera Sciences. Dr. Coughlin is an employee of Alimera Sciences. Dr. Riemann received financial support from AGTC, Alcon, Alimera Sciences, Allergan, Arepio, Chengdu Kanghong, Clearside, Genentech/Roche, Gyroscope, Janssen/Johnson & Johnson, Lineage (Formerly BioTime), Lowry-MacTel Registry, Neurotech, Nightstar/Biogen, NotalVision, Novartis, Ophthotec/Iveric, Regeneron, RegenexBio, and Spark; is a consultant to Alcon, Alimera, Alimera Deutschland GmBH, Allergan, Animal Eye Institute, Bausch + Lomb/Valeant, BMC/Eyetube, DORC, Genentech/Roche, Gore, Gyroscope, Haag Streit AG, Haag Streit Surgical, Haag Streit USA, HumanOptics AG, Iamc2, iVeena, Janssen/Johnson & Johnson, Kaleidoscope Engineering, Lineage (Formerly BioTime), MedOne, Neuracle, NotalVision, Orbit BioMedical, RegenexBio, Reliance Industries, SalutarisMD, Samsara, TrueVision, Vortex Surgical, and Zeiss; is a lecturer for Alcon, Alimera Sciences, Alimera Deutschland GmBH, Allergan, Bausch + Lomb/Valeant, CSTLII, Novartis, Regeneron, Reliance Industries, SalutarisMD, and TrueVision; owns equity in CVP (CEI Vision Partners)/ECP (Eye Care Partners), Digital Surgery Systems, ForwardVue Pharma, iVeena, TrueVision, and Vortex Surgical; receives royalties from MedOne, TrueVision, and Vortex Surgical; owns intellectual property with Haag Streit USA, Iamc2, Janssen/Johnson & Johnson, Kaleidoscope Engineering, MedOne, Reliance Industries; and has leadership roles with Aniridia Foundation International (Medical and Scientific Advisory Council), Chruman Research (Owner/Cofounder), Clovernook Center for the Blind and Visually Impaired (Board of Trustees), CVP (CEI Vision Partners)/ECP (Eye Care Partners) (Owner/Cofounder), Digital Surgery Systems (Owner), ForwardVue Pharma (Board of Directors), Iamc2 (Cofounder), Macor Industries (Owner), Northmark Pharmacy (Owner), and VEO (Owner/Cofounder).
Figures
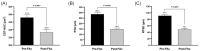

References
-
- Duphare C, Desai K, Gupta P, Patel BC. Diabetic macular edema. In: StatPearls. StatPearls Publishing; 2022. - PubMed
-
- Starr MR, Salabati M, Mahmoudzadeh R, et al. Fluctuations in central subfield thickness associated with worse visual outcomes in patients with diabetic macular edema in clinical trial setting. Am J Ophthalmol. 2021;232:90-97. - PubMed
-
- Riemann CD, Eaton AM, Cutino A. Reduction in retinal thickness fluctuations after treatment with fluocinolone acetonide implant for DME: a post-hoc analysis of the USER study. Ophthalmic Surg Lasers Imaging Retina. 2020;51(5):298-306. - PubMed
LinkOut - more resources
Full Text Sources

