Phthalocyanine aggregates in the photodynamic therapy: dogmas, controversies, and future prospects
- PMID: 37975002
- PMCID: PMC10643719
- DOI: 10.1007/s12551-023-01129-7
Phthalocyanine aggregates in the photodynamic therapy: dogmas, controversies, and future prospects
Abstract
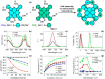
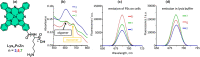
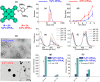
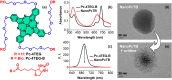
Photodynamic therapy (PDT), a rapidly developing method for the treatment of cancer and bacterial diseases, is based on the photosensitization of oxygen to generate reactive oxygen species (ROS) that destroy specific biological targets. Among the various photosensitizers, phthalocyanines (Pc) have attracted particular attention due to their excellent photophysical properties, most of which meet the therapeutic requirements. The statement that aggregation of Pc-based photosensitizers is undesirable because it suppresses ROS generation has become commonplace in PDT. In this review, we have collected and discussed a number of works whose results refute this well-established axiom and show that aggregated forms of phthalocyanines can still exhibit photodynamic activity, in some cases in synergy with the photothermal and optoacoustic effects. In addition, ROS generation can be induced by aggregates under the conditions of sonodynamic therapy.
Keywords: Nanocarrier; Nanomedicine; Photodynamic therapy; Photophysics; Reactive oxygen species; Self-assembly.
© International Union for Pure and Applied Biophysics (IUPAB) and Springer-Verlag GmbH Germany, part of Springer Nature 2023. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures




References
-
- Bandera Y, Burdette MK, Shetzline JA, et al. Synthesis of water soluble axially disubstituted silicon (IV) phthalocyanines with alkyne & azide functionality. Dye Pigment. 2016;125:72–79. doi: 10.1016/j.dyepig.2015.10.007. - DOI
Publication types
LinkOut - more resources
Full Text Sources

