Directed proton transfer from Fo to F1 extends the multifaceted proton functions in ATP synthase
- PMID: 37975013
- PMCID: PMC10643803
- DOI: 10.1007/s12551-023-01132-y
Directed proton transfer from Fo to F1 extends the multifaceted proton functions in ATP synthase
Abstract
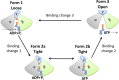
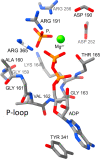
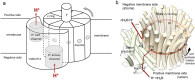
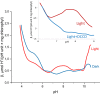
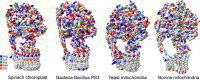
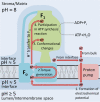
The role of protons in ATP synthase is typically considered to be energy storage in the form of an electrochemical potential, as well as an operating element proving rotation. However, this review emphasizes that protons also act as activators of conformational changes in F1 and as direct participants in phosphorylation reaction. The protons transferred through Fo do not immediately leave to the bulk aqueous phase, but instead provide for the formation of a pH gradient between acidifying Fo and alkalizing F1. It facilitates a directed inter-subunit proton transfer to F1, where they are used in the ATP synthesis reaction. This ensures that the enzyme activity is not limited by a lack of protons in the alkaline mitochondrial matrix or chloroplast stroma. Up to one hundred protons bind to the carboxyl groups of the F1 subunit, altering the electrical interactions between the amino acids of the enzyme. This removes the inhibition of ATP synthase caused by the electrostatic attraction of charged amino acids of the stator and rotor and also makes the enzyme more prone to conformational changes. Protonation occurs during ATP synthesis initiation and during phosphorylation, while deprotonation blocks the rotation inhibiting both synthesis and hydrolysis. Thus, protons participate in the functioning of all main components of ATP synthase molecular machine making it effectively a proton-driven electric machine. The review highlights the key role of protons as a coupling factor in ATP synthase with multifaceted functions, including charge and energy transport, torque generation, facilitation of conformational changes, and participation in the ATP synthesis reaction.
Keywords: F1Fo ATP synthase; H+ ions; Oxidative phosphorylation system; Protein conformational changes; Proton transport.
© International Union for Pure and Applied Biophysics (IUPAB) and Springer-Verlag GmbH Germany, part of Springer Nature 2023. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures

Similar articles
-
F1FO ATP synthase molecular motor mechanisms.Front Microbiol. 2022 Aug 23;13:965620. doi: 10.3389/fmicb.2022.965620. eCollection 2022. Front Microbiol. 2022. PMID: 36081786 Free PMC article. Review.
-
pH-dependent 11° F1FO ATP synthase sub-steps reveal insight into the FO torque generating mechanism.Elife. 2021 Dec 31;10:e70016. doi: 10.7554/eLife.70016. Elife. 2021. PMID: 34970963 Free PMC article.
-
Mutational analysis of a conserved positive charge in the c-ring of E. coli ATP synthase.Biochim Biophys Acta Bioenerg. 2023 Apr 1;1864(2):148962. doi: 10.1016/j.bbabio.2023.148962. Epub 2023 Feb 21. Biochim Biophys Acta Bioenerg. 2023. PMID: 36822493 Free PMC article.
-
The a subunit asymmetry dictates the two opposite rotation directions in the synthesis and hydrolysis of ATP by the mitochondrial ATP synthase.Med Hypotheses. 2015 Jan;84(1):53-7. doi: 10.1016/j.mehy.2014.11.015. Epub 2014 Dec 2. Med Hypotheses. 2015. PMID: 25497387
-
Rotational Mechanism of FO Motor in the F-Type ATP Synthase Driven by the Proton Motive Force.Front Microbiol. 2022 Jun 16;13:872565. doi: 10.3389/fmicb.2022.872565. eCollection 2022. Front Microbiol. 2022. PMID: 35783438 Free PMC article. Review.
Cited by
-
Cobra Venom Cytotoxins as a Tool for Probing Mechanisms of Mitochondrial Energetics and Understanding Mitochondrial Membrane Structure.Toxins (Basel). 2024 Jun 25;16(7):287. doi: 10.3390/toxins16070287. Toxins (Basel). 2024. PMID: 39057927 Free PMC article. Review.
-
VII Congress of Russian Biophysicists-2023, Krasnodar, Russia.Biophys Rev. 2023 Oct 13;15(5):801-805. doi: 10.1007/s12551-023-01164-4. eCollection 2023 Oct. Biophys Rev. 2023. PMID: 37975012 Free PMC article.
-
Calcium signaling in postsynaptic mitochondria: mechanisms, dynamics, and role in ATP production.Front Mol Neurosci. 2025 Jul 21;18:1621070. doi: 10.3389/fnmol.2025.1621070. eCollection 2025. Front Mol Neurosci. 2025. PMID: 40761376 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

