Intravenous BCAA Infusion Does Not Lead to a Sustained Recovery From Overt HE in ACLF - An Open Label Randomized Clinical Trial
- PMID: 37975059
- PMCID: PMC10643498
- DOI: 10.1016/j.jceh.2023.05.015
Intravenous BCAA Infusion Does Not Lead to a Sustained Recovery From Overt HE in ACLF - An Open Label Randomized Clinical Trial
Abstract
Background: Hepatic encephalopathy (HE) in acute-on-chronic liver failure (ACLF) is associated with significant morbidity and mortality. We conducted a prospective, randomized controlled clinical trial to study the efficacy of intravenous branched chain amino acids (IV-BCAA) with lactulose versus lactulose alone for improvement in HE at 24 h, day 3, and day 7. The primary outcome was an improvement in encephalopathy by ≥ 1 grade at 72 h.
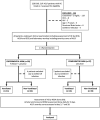
Patients and methods: European association for study of liver (EASL) defined ACLF patients with overt HE were assessed and randomized into the experimental arm (IV-BCAA - 500 mL/day for 3 days + Lactulose; n = 39) and the comparator arm (Lactulose alone; n = 37). Six patients developed COVID-19 after randomization and were excluded (4-experimental arm and 2-comparator arm).
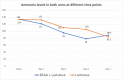
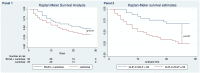
Results: Of 222 screened patients, 70 (35 in each arm) were included in the analysis. Baseline characteristics, including HE grade (2.9 ± 0.7 vs 2.8 ± 0.7; P = 0.86) and (chronic liver failure) CLIF-C ACLF score (54.2 ± 5.6 vs 54.8 ± 5.7; P = 0.65), were similar. Overall survival was 40% at 28 days (48.5% vs 31.4%; P = 0.14). Improvement in hepatic encephalopathy scoring algorithm (HESA) by ≥ 1 grade at 24 h occurred in 14 patients (40%) in the BCAA arm and 6 patients (17.1%) in the control group (P = 0.03) which translated to a shorter intensive care unit (ICU) stay. The median change in HESA at 24 h was greater in the BCAA arm than the control arm (P = 0.006), which was not sustained at days 3 or 7. Ammonia levels did not correlate with the grade of HE (Spearman's correlation coefficient (ρ) = - 0.0843; P = 0.29).
Conclusion: Intravenous BCAA does not lead to a sustained improvement in HE grade in ACLF.
Trial registration no: NCT04238416 (clinicaltrials.gov).
Keywords: acute-on-chronic liver failure; branched chain amino acids; hepatic encephalopathy; lactulose.
© 2023 Indian National Association for Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures
References
-
- Cordoba J., Ventura-Cots M., Simón-Talero M., et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute-on-chronic liver failure. J Hepatol. 2014;60:275–281. - PubMed
-
- López-Vicario C., Checa A., Urdangarin A., et al. Targeted lipidomics reveals extensive changes in circulating lipid mediators in patients with acutely decompensated cirrhosis. J Hepatol. 2020;73:817–828. - PubMed
-
- Bosoi C.R., Rose C.F. Brain edema in acute liver failure and chronic liver disease: similarities and differences. Neurochem Int. 2013 Mar;62:446–457. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

