Cytomegalovirus (CMV) Reactivation and CMV-Specific Cell-Mediated Immunity After Chimeric Antigen Receptor T-Cell Therapy
- PMID: 37975819
- PMCID: PMC11006113
- DOI: 10.1093/cid/ciad708
Cytomegalovirus (CMV) Reactivation and CMV-Specific Cell-Mediated Immunity After Chimeric Antigen Receptor T-Cell Therapy
Abstract
Background: The epidemiology of cytomegalovirus (CMV) after chimeric antigen receptor-modified T-cell immunotherapy (CARTx) is poorly understood owing to a lack of routine surveillance.
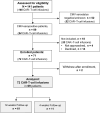
Methods: We prospectively enrolled 72 adult CMV-seropositive CD19-, CD20-, or BCMA-targeted CARTx recipients and tested plasma samples for CMV before and weekly up to 12 weeks after CARTx. We assessed CMV-specific cell-mediated immunity (CMV-CMI) before and 2 and 4 weeks after CARTx, using an interferon γ release assay to quantify T-cell responses to IE-1 and pp65. We tested pre-CARTx samples to calculate a risk score for cytopenias and infection (CAR-HEMATOTOX). We used Cox regression to evaluate CMV risk factors and evaluated the predictive performance of CMV-CMI for CMV reactivation in receiver operator characteristic curves.
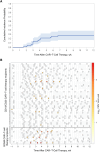
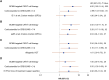
Results: CMV was detected in 1 patient (1.4%) before and in 18 (25%) after CARTx, for a cumulative incidence of 27% (95% confidence interval, 16.8-38.2). The median CMV viral load (interquartile range) was 127 (interquartile range, 61-276) IU/mL, with no end-organ disease observed; 5 patients received preemptive therapy based on clinical results. CMV-CMI values reached a nadir 2 weeks after infusion and recovered to baseline levels by week 4. In adjusted models, BCMA-CARTx (vs CD19/CD20) and corticosteroid use for >3 days were significantly associated with CMV reactivation, and possible associations were detected for lower week 2 CMV-CMI and more prior antitumor regimens. The cumulative incidence of CMV reactivation almost doubled when stratified by BCMA-CARTx target and use of corticosteroids for >3 days (46% and 49%, respectively).
Conclusions: CMV testing could be considered between 2 and 6 weeks in high-risk CARTx recipients.
Keywords: CAR; CMV; chimeric antigen receptor; cytomegalovirus; immunotherapy.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. E. K. reports travel support from Merck to attend the International Immunocompromised Host Society (ICHS) symposium 2022. D. J. G. has served as an advisor and has received research funding and royalties from Juno Therapeutics, a Bristol-Myers Squibb company; has served as an advisor and received research funding from Seattle Genetics; has served as an advisor for GlaxoSmithKline, Celgene, Ensoma, Janssen Biotech, and Legend Biotech; and has received research funding from SpringWorks Therapeutics, Sanofi, and Cellectar Biosciences. J. G. has served as ad hoc consultant and has received honoraria from Sobi, Legend Biotech, Janssen, Kite Pharma, and MorphoSys; has received research funding from Sobi, Juno Therapeutics (a Bristol-Myers Squibb company), Celgene (a Bristol-Myers Squibb company), and Angiocrine Bioscience; and has participated in the independent data review committee for Century Therapeutics. D. G. M. has served as ad hoc consultant and has received honoraria from BMS, Celgene, Genentech, Juno Therapeutics, and Kite. D. G. M.'s institution, Fred Hutchinson Cancer Center, has received research funding, including salary support, from the following companies for clinical trials on which D. G. M. is a principal investigator or subinvestigator: Kite Pharma, Juno Therapeutics, Celgene, Legend Biotech, and BMS. D. G. M. has the rights to royalties from the Fred Hutchinson Cancer Center for patents licensed to Juno Therapeutics/BMS; has stock options from A2 Biotherapeutics and Navan Technologies; has served as member with compensation for A2 Biotherapeutics, Navan, and Chimeric Therapeutics (member of the Scientific Advisory Board), Genentech (member and chair of the Lymphoma Steering Committee), BMS (member of the JCAR017 EAP-001 Safety Review Committee), BMS (member of the CLL Strategic Council), ImmPACT Bio (member of the Clinical Advisory Board and the CD19/CD20 bispecific CAR-T Cell Therapy Program), Gilead Sciences (member of the Scientific Review Committee and Research Scholars Program in Hematologic Malignancies), and Interius (member of Clinical Advisory Board); has served as member without compensation for BMS (member of the JCAR017-BCM-03 Scientific Steering Committee). M. J. B has served as consultant and has received research funding from Merck; has served as consultant for Symbio, Helocyte, Moderna, and Allovir; and has served as a consultant and had option to acquire stock for EvrysBio. J. A. H. has served as a consultant for Moderna, Allovir, Gilead, SentiBio, Modulus, and Allogene and received research funding from Allovir, Gilead, and Merck. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



References
-
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2019; 380:45–56. - PubMed
-
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 2020; 396:839–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

