Mitochondrial aminoacyl-tRNA synthetases trigger unique compensatory mechanisms in neurons
- PMID: 37975900
- PMCID: PMC10877469
- DOI: 10.1093/hmg/ddad196
Mitochondrial aminoacyl-tRNA synthetases trigger unique compensatory mechanisms in neurons
Abstract
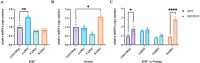
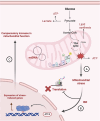
Mitochondrial aminoacyl-tRNA synthetase (mt-ARS) mutations cause severe, progressive, and often lethal diseases with highly heterogeneous and tissue-specific clinical manifestations. This study investigates the molecular mechanisms triggered by three different mt-ARS defects caused by biallelic mutations in AARS2, EARS2, and RARS2, using an in vitro model of human neuronal cells. We report distinct molecular mechanisms of mitochondrial dysfunction among the mt-ARS defects studied. Our findings highlight the ability of proliferating neuronal progenitor cells (iNPCs) to compensate for mitochondrial translation defects and maintain balanced levels of oxidative phosphorylation (OXPHOS) components, which becomes more challenging in mature neurons. Mutant iNPCs exhibit unique compensatory mechanisms, involving specific branches of the integrated stress response, which may be gene-specific or related to the severity of the mitochondrial translation defect. RNA sequencing revealed distinct transcriptomic profiles showing dysregulation of neuronal differentiation and protein translation. This study provides valuable insights into the tissue-specific compensatory mechanisms potentially underlying the phenotypes of patients with mt-ARS defects. Our novel in vitro model may more accurately represent the neurological presentation of patients and offer an improved platform for future investigations and therapeutic development.
Keywords: aminoacyl-tRNA synthetase; mitochondrial biology; neurological disease; protein synthesis.
© The Author(s) 2023. Published by Oxford University Press.
Figures