Higher-Dose Fluvoxamine and Time to Sustained Recovery in Outpatients With COVID-19: The ACTIV-6 Randomized Clinical Trial
- PMID: 37976072
- PMCID: PMC10656670
- DOI: 10.1001/jama.2023.23363
Higher-Dose Fluvoxamine and Time to Sustained Recovery in Outpatients With COVID-19: The ACTIV-6 Randomized Clinical Trial
Abstract
Importance: The effect of higher-dose fluvoxamine in reducing symptom duration among outpatients with mild to moderate COVID-19 remains uncertain.
Objective: To assess the effectiveness of fluvoxamine, 100 mg twice daily, compared with placebo, for treating mild to moderate COVID-19.
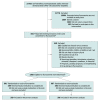
Design, setting, and participants: The ACTIV-6 platform randomized clinical trial aims to evaluate repurposed medications for mild to moderate COVID-19. Between August 25, 2022, and January 20, 2023, a total of 1175 participants were enrolled at 103 US sites for evaluating fluvoxamine; participants were 30 years or older with confirmed SARS-CoV-2 infection and at least 2 acute COVID-19 symptoms for 7 days or less.
Interventions: Participants were randomized to receive fluvoxamine, 50 mg twice daily on day 1 followed by 100 mg twice daily for 12 additional days (n = 601), or placebo (n = 607).
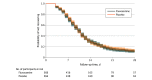
Main outcomes and measures: The primary outcome was time to sustained recovery (defined as at least 3 consecutive days without symptoms). Secondary outcomes included time to death; time to hospitalization or death; a composite of hospitalization, urgent care visit, emergency department visit, or death; COVID-19 clinical progression scale score; and difference in mean time unwell. Follow-up occurred through day 28.
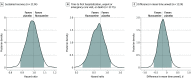
Results: Among 1208 participants who were randomized and received the study drug, the median (IQR) age was 50 (40-60) years, 65.8% were women, 45.5% identified as Hispanic/Latino, and 76.8% reported receiving at least 2 doses of a SARS-CoV-2 vaccine. Among 589 participants who received fluvoxamine and 586 who received placebo included in the primary analysis, differences in time to sustained recovery were not observed (adjusted hazard ratio [HR], 0.99 [95% credible interval, 0.89-1.09]; P for efficacy = .40]). Additionally, unadjusted median time to sustained recovery was 10 (95% CI, 10-11) days in both the intervention and placebo groups. No deaths were reported. Thirty-five participants reported health care use events (a priori defined as death, hospitalization, or emergency department/urgent care visit): 14 in the fluvoxamine group compared with 21 in the placebo group (HR, 0.69 [95% credible interval, 0.27-1.21]; P for efficacy = .86) There were 7 serious adverse events in 6 participants (2 with fluvoxamine and 4 with placebo) but no deaths.
Conclusions and relevance: Among outpatients with mild to moderate COVID-19, treatment with fluvoxamine does not reduce duration of COVID-19 symptoms.
Trial registration: ClinicalTrials.gov Identifier: NCT04885530.
Conflict of interest statement
Figures
Comment in
-
Learning From the Success of the ACTIV Platform.JAMA. 2023 Dec 26;330(24):2363-2364. doi: 10.1001/jama.2023.24087. JAMA. 2023. PMID: 37976051 No abstract available.
References
-
- COVID-19 Treatment Guidelines . National Institutes of Health. Accessed September 5, 2023. https://www.covid19treatmentguidelines.nih.gov/
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

