ZmEREB92 plays a negative role in seed germination by regulating ethylene signaling and starch mobilization in maize
- PMID: 37976306
- PMCID: PMC10691696
- DOI: 10.1371/journal.pgen.1011052
ZmEREB92 plays a negative role in seed germination by regulating ethylene signaling and starch mobilization in maize
Abstract
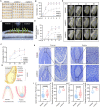
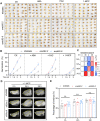
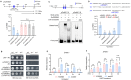
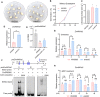
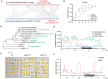
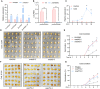
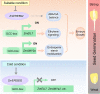
Rapid and uniform seed germination is required for modern cropping system. Thus, it is important to optimize germination performance through breeding strategies in maize, in which identification for key regulators is needed. Here, we characterized an AP2/ERF transcription factor, ZmEREB92, as a negative regulator of seed germination in maize. Enhanced germination in ereb92 mutants is contributed by elevated ethylene signaling and starch degradation. Consistently, an ethylene signaling gene ZmEIL7 and an α-amylase gene ZmAMYa2 are identified as direct targets repressed by ZmEREB92. OsERF74, the rice ortholog of ZmEREB92, shows conserved function in negatively regulating seed germination in rice. Importantly, this orthologous gene pair is likely experienced convergently selection during maize and rice domestication. Besides, mutation of ZmEREB92 and OsERF74 both lead to enhanced germination under cold condition, suggesting their regulation on seed germination might be coupled with temperature sensitivity. Collectively, our findings uncovered the ZmEREB92-mediated regulatory mechanism of seed germination in maize and provide breeding targets for maize and rice to optimize seed germination performance towards changing climates.
Copyright: © 2023 Fu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Identification of Quantitative Trait Loci Controlling Ethylene Production in Germinating Seeds in Maize (Zea mays L.).Sci Rep. 2020 Feb 3;10(1):1677. doi: 10.1038/s41598-020-58607-1. Sci Rep. 2020. PMID: 32015470 Free PMC article.
-
ZmEREB92 interacts with ZmMYC2 to activate maize terpenoid phytoalexin biosynthesis upon Fusarium graminearum infection through jasmonic acid/ethylene signaling.New Phytol. 2023 Feb;237(4):1302-1319. doi: 10.1111/nph.18590. Epub 2022 Dec 8. New Phytol. 2023. PMID: 36319608
-
Analysis of gene expression in early seed germination of rice: landscape and genetic regulation.BMC Plant Biol. 2022 Feb 17;22(1):70. doi: 10.1186/s12870-022-03458-3. BMC Plant Biol. 2022. PMID: 35176996 Free PMC article.
-
The Rice Alpha-Amylase, Conserved Regulator of Seed Maturation and Germination.Int J Mol Sci. 2019 Jan 21;20(2):450. doi: 10.3390/ijms20020450. Int J Mol Sci. 2019. PMID: 30669630 Free PMC article. Review.
-
Advances of Apetala2/Ethylene Response Factors in Regulating Development and Stress Response in Maize.Int J Mol Sci. 2023 Mar 12;24(6):5416. doi: 10.3390/ijms24065416. Int J Mol Sci. 2023. PMID: 36982510 Free PMC article. Review.
Cited by
-
The Diterpene Isopimaric Acid Modulates the Phytohormone Pathway to Promote Oryza sativa L. Rice Seedling Growth.Curr Issues Mol Biol. 2024 Sep 2;46(9):9772-9784. doi: 10.3390/cimb46090580. Curr Issues Mol Biol. 2024. PMID: 39329932 Free PMC article.
-
Molecular Characteristics and Functional Identification of a Key Alpha-Amylase-Encoding Gene AMY11 in Musa acuminata.Int J Mol Sci. 2024 Jul 17;25(14):7832. doi: 10.3390/ijms25147832. Int J Mol Sci. 2024. PMID: 39063074 Free PMC article.
-
Ethylene Signaling in Regulating Plant Growth, Development, and Stress Responses.Plants (Basel). 2025 Jan 21;14(3):309. doi: 10.3390/plants14030309. Plants (Basel). 2025. PMID: 39942870 Free PMC article. Review.
-
A Critical Review of Recent Advances in Maize Stress Molecular Biology.Int J Mol Sci. 2024 Nov 18;25(22):12383. doi: 10.3390/ijms252212383. Int J Mol Sci. 2024. PMID: 39596447 Free PMC article. Review.
References
-
- Greaves JA. Improving suboptimal temperature tolerance in maize. J Exp Bot. 1996; 47: 307–323 doi: 10.1093/jxb/47.3.307 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

