Zona incerta dopamine neurons encode motivational vigor in food seeking
- PMID: 37976360
- PMCID: PMC10656063
- DOI: 10.1126/sciadv.adi5326
Zona incerta dopamine neurons encode motivational vigor in food seeking
Abstract
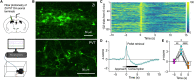
Energy deprivation triggers food seeking to ensure homeostatic consumption, but the neural coding of motivational vigor in food seeking during physical hunger remains unknown. Here, we report that ablation of dopamine (DA) neurons in zona incerta (ZI) but not ventral tegmental area potently impaired food seeking after fasting. ZI DA neurons and their projections to paraventricular thalamus (PVT) were quickly activated for food approach but inhibited during food consumption. Chemogenetic manipulation of ZI DA neurons bidirectionally regulated feeding motivation to control meal frequency but not meal size for food intake. Activation of ZI DA neurons promoted, but silencing of these neurons blocked, contextual memory associate with food reward. In addition, selective activation of ZI DA projections to PVT promoted food seeking for food consumption and transited positive-valence signals. Together, these findings reveal that ZI DA neurons encode motivational vigor in food seeking for food consumption through their projections to PVT.
Figures






Update of
-
Zona incerta dopamine neurons encode motivational vigor in food seeking.bioRxiv [Preprint]. 2023 Jun 29:2023.06.29.547060. doi: 10.1101/2023.06.29.547060. bioRxiv. 2023. Update in: Sci Adv. 2023 Nov 15;9(46):eadi5326. doi: 10.1126/sciadv.adi5326. PMID: 37425830 Free PMC article. Updated. Preprint.
Similar articles
-
Zona incerta dopamine neurons encode motivational vigor in food seeking.bioRxiv [Preprint]. 2023 Jun 29:2023.06.29.547060. doi: 10.1101/2023.06.29.547060. bioRxiv. 2023. Update in: Sci Adv. 2023 Nov 15;9(46):eadi5326. doi: 10.1126/sciadv.adi5326. PMID: 37425830 Free PMC article. Updated. Preprint.
-
Zona incerta neurons projecting to the ventral tegmental area promote action initiation towards feeding.J Physiol. 2021 Jan;599(2):709-724. doi: 10.1113/JP276513. Epub 2020 Dec 22. J Physiol. 2021. PMID: 33296086 Free PMC article.
-
Serotonin activates paraventricular thalamic neurons through direct depolarization and indirect disinhibition from zona incerta.J Physiol. 2021 Nov;599(21):4883-4900. doi: 10.1113/JP282088. Epub 2021 Sep 23. J Physiol. 2021. PMID: 34510418
-
Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat: Historical perspective.Brain Res. 2016 Aug 15;1645:68-70. doi: 10.1016/j.brainres.2015.12.041. Epub 2015 Dec 28. Brain Res. 2016. PMID: 26731335 Review.
-
Zona Incerta: An Integrative Node for Global Behavioral Modulation.Trends Neurosci. 2020 Feb;43(2):82-87. doi: 10.1016/j.tins.2019.11.007. Epub 2019 Dec 18. Trends Neurosci. 2020. PMID: 31864676 Free PMC article. Review.
Cited by
-
Regulation of PVT-CeA Circuit in Deoxynivalenol-Induced Anorexia and Aversive-Like Emotions.Adv Sci (Weinh). 2025 Apr;12(16):e2417068. doi: 10.1002/advs.202417068. Epub 2025 Feb 28. Adv Sci (Weinh). 2025. PMID: 40019402 Free PMC article.
-
Novel neural pathways targeted by GLP-1R agonists and bariatric surgery.Pflugers Arch. 2025 Feb;477(2):171-185. doi: 10.1007/s00424-024-03047-3. Epub 2024 Dec 7. Pflugers Arch. 2025. PMID: 39644359 Free PMC article. Review.
-
Unilateral and Bilateral Subthalamic Deep Brain Stimulation Differently Favour Apathy in Parkinson's Disease.Eur J Neurosci. 2025 Feb;61(4):e70019. doi: 10.1111/ejn.70019. Eur J Neurosci. 2025. PMID: 39962903 Free PMC article.
-
Decoding gene networks controlling hypothalamic and prethalamic neuron development.Cell Rep. 2025 Jun 24;44(6):115858. doi: 10.1016/j.celrep.2025.115858. Epub 2025 Jun 12. Cell Rep. 2025. PMID: 40512619 Free PMC article.
-
Multiple cholinergic receptor subtypes coordinate dual modulation of acetylcholine on anterior and posterior paraventricular thalamic neurons.J Neurochem. 2024 Jun;168(6):995-1018. doi: 10.1111/jnc.16115. Epub 2024 Apr 25. J Neurochem. 2024. PMID: 38664195 Free PMC article.
References
-
- Q. Y. Zhou, R. D. Palmiter, Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 83, 1197–1209 (1995). - PubMed
-
- L. Boekhoudt, T. J. M. Roelofs, J. W. de Jong, A. E. de Leeuw, M. C. M. Luijendijk, I. G. Wolterink-Donselaar, G. van der Plasse, R. A. H. Adan, Does activation of midbrain dopamine neurons promote or reduce feeding? Int. J. Obes. (Lond) 41, 1131–1140 (2017). - PubMed
-
- R. D. Palmiter, Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 30, 375–381 (2007). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

