Fixing the Leaky Pipe: How to Improve the Uptake of Patient-Reported Outcomes-Based Prognostic and Predictive Models in Cancer Clinical Practice
- PMID: 37976441
- PMCID: PMC10681558
- DOI: 10.1200/CCI.23.00070
Fixing the Leaky Pipe: How to Improve the Uptake of Patient-Reported Outcomes-Based Prognostic and Predictive Models in Cancer Clinical Practice
Abstract
Purpose: This discussion paper outlines challenges and proposes solutions for successfully implementing prediction models that incorporate patient-reported outcomes (PROs) in cancer practice.
Methods: We organized a full-day multidisciplinary meeting of people with expertise in cancer care delivery, PRO collection, PRO use in prediction modeling, computing, implementation, and decision science. The discussions presented here focused on identifying challenges to the development, implementation and use of prediction models incorporating PROs, and suggesting possible solutions.
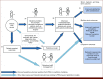
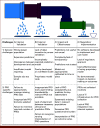
Results: Specific challenges and solutions were identified across three broad areas. (1) Understanding decision making and implementation: necessitating multidisciplinary collaboration in the early stages and throughout; early stakeholder engagement to define the decision problem and ensure acceptability of PROs in prediction; understanding patient/clinician interpretation of PRO predictions and uncertainty to optimize prediction impact; striving for model integration into existing electronic health records; and early regulatory alignment. (2) Recognizing the limitations to PRO collection and their impact on prediction: incorporating validated, clinically important PROs to maximize model generalizability and clinical engagement; and minimizing missing PRO data (resulting from both structural digital exclusion and time-varying factors) to avoid exacerbating existing inequalities. (3) Statistical and modeling challenges: incorporating statistical methods to address missing data; ensuring predictive modeling recognizes complex causal relationships; and considering temporal and geographic recalibration so that model predictions reflect the relevant population.
Conclusion: Developing and implementing PRO-based prediction models in cancer care requires extensive multidisciplinary working from the earliest stages, recognition of implementation challenges because of PRO collection and model presentation, and robust statistical methods to manage missing data, causality, and calibration. Prediction models incorporating PROs should be viewed as complex interventions, with their development and impact assessment carried out to reflect this.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
References
-
- Rothwell PM: External validity of randomised controlled trials: “To whom do the results of this trial apply?” Lancet 365:82-93, 2005 - PubMed
-
- Harding R Simms V Calanzani N, et al. : If you had less than a year to live, would you want to know? A seven-country European population survey of public preferences for disclosure of poor prognosis. Psychooncology 22:2298-2305, 2013 - PubMed
-
- Weeks JC Cook EF O’Day SJ, et al. : Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA 279:1709-1714, 1998 - PubMed
-
- Temel JS Greer JA Admane S, et al. : Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non–small-cell lung cancer: Results of a randomized study of early palliative care. J Clin Oncol 29:2319-2326, 2011 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

