SKYSCRAPER-02: Tiragolumab in Combination With Atezolizumab Plus Chemotherapy in Untreated Extensive-Stage Small-Cell Lung Cancer
- PMID: 37976444
- PMCID: PMC10824371
- DOI: 10.1200/JCO.23.01363
SKYSCRAPER-02: Tiragolumab in Combination With Atezolizumab Plus Chemotherapy in Untreated Extensive-Stage Small-Cell Lung Cancer
Abstract
Purpose: The phase III SKYSCRAPER-02 study determined whether the benefits of atezolizumab plus carboplatin and etoposide (CE) could be enhanced by the addition of tiragolumab in untreated extensive-stage small-cell lung cancer (ES-SCLC). We report final progression-free survival (PFS) and overall survival (OS) analyses.
Methods: Patients received tiragolumab 600 mg/placebo, plus atezolizumab 1,200 mg and CE (four cycles), then maintenance tiragolumab/placebo plus atezolizumab. Primary end points were investigator-assessed PFS and OS in patients without history/presence of brain metastases (primary analysis set [PAS]). Additional end points included PFS and OS in all patients regardless of brain metastases status (full analysis set [FAS]), response, and safety.
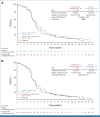
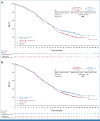
Results: Four hundred ninety patients were randomly assigned (FAS): 243 to tiragolumab arm and 247 to control arm. At the cutoff date (February 6, 2022; median duration of follow-up, 14.3 months [PAS] and 13.9 months [FAS]), final analysis of PFS in the PAS (n = 397) did not reach statistical significance (stratified hazard ratio [HR], 1.11; P = .3504; median, 5.4 months tiragolumab v 5.6 months control). At the cutoff date (September 6, 2022; median duration of follow-up, 21.2 months [FAS]), median OS in the PAS at final OS analysis was 13.1 months in both arms (stratified HR, 1.14; P = .2859). Median PFS and OS in the FAS were consistent with the PAS. The proportion of patients with immune-mediated adverse events (AEs) in the tiragolumab and control arms was 54.4% and 49.2%, respectively (grade 3/4: 7.9% and 7.7%). AEs leading to treatment withdrawal occurred in 8.4% and 9.3% of tiragolumab- and control-treated patients, respectively.
Conclusion: Tiragolumab did not provide additional benefit over atezolizumab and CE in untreated ES-SCLC. The combination was well tolerated with no new safety signals.
Trial registration: ClinicalTrials.gov NCT04256421.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- Horn L, Mansfield AS, Szczęsna A, et al. : First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379:2220-2229, 2018 - PubMed
-
- Clinical Practice Guidelines in Oncology: Small Cell Lung Cancer. 2022. http://NCCN.org
-
- Paz-Ares L, Dvorkin M, Chen Y, et al. : Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 394:1929-1939, 2019 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

