Avelumab first-line maintenance treatment for advanced urothelial carcinoma: review of evidence to guide clinical practice
- PMID: 37976999
- PMCID: PMC10685024
- DOI: 10.1016/j.esmoop.2023.102050
Avelumab first-line maintenance treatment for advanced urothelial carcinoma: review of evidence to guide clinical practice
Abstract
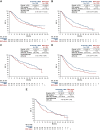
The JAVELIN Bladder 100 phase III trial led to the incorporation of avelumab first-line (1L) maintenance treatment into international guidelines as a standard of care for patients with advanced urothelial carcinoma (UC) without progression after 1L platinum-based chemotherapy. JAVELIN Bladder 100 showed that avelumab 1L maintenance significantly prolonged overall survival (OS) and progression-free survival in this population compared with a 'watch-and-wait' approach. The aim of this manuscript is to review clinical studies of avelumab 1L maintenance in patients with advanced UC, including long-term efficacy and safety data from JAVELIN Bladder 100, subgroup analyses in clinically relevant subpopulations, and 'real-world' data obtained outside of clinical trials, providing a comprehensive resource to support patient management. Extended follow-up from JAVELIN Bladder 100 has shown that avelumab provides a long-term efficacy benefit, with a median OS of 23.8 months measured from start of maintenance treatment, and 29.7 months measured from start of 1L chemotherapy. Longer OS was observed across subgroups, including patients who received 1L cisplatin + gemcitabine, patients who received four or six cycles of 1L chemotherapy, and patients with complete response, partial response, or stable disease as best response to 1L induction chemotherapy. No new safety signals were seen in patients who received ≥1 year of avelumab treatment, and toxicity was similar in those who had received cisplatin or carboplatin with gemcitabine. Other clinical datasets, including noninterventional studies conducted in Europe, USA, and Asia, have confirmed the efficacy of avelumab 1L maintenance. Potential subsequent treatment options after avelumab maintenance include antibody-drug conjugates (enfortumab vedotin or sacituzumab govitecan), erdafitinib in biomarker-selected patients, platinum rechallenge in suitable patients, nonplatinum chemotherapy, and clinical trial participation; however, evidence to determine optimal treatment sequences is needed. Ongoing trials of avelumab-based combination regimens as maintenance treatment have the potential to evolve the treatment landscape for patients with advanced UC.
Keywords: avelumab; bladder cancer; first line; maintenance treatment; urothelial carcinoma.
Copyright © 2023 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
PG has served in consulting or advisory roles for 4D Pharma, Aadi Bioscience, Asieris Pharmaceuticals, Astellas, AstraZeneca, BostonGene, Bristol Myers Squibb, CG Oncology, Dyania Health, Exelixis, Fresenius Kabi, G1 Therapeutics, Genentech, Gilead Sciences, Guardant Health, ImmunityBio, Infinity Pharmaceuticals, Janssen, Lucence, Merck, Mirati Therapeutics, MSD, Pfizer, PureTech, QED Therapeutics, Regeneron, Roche, Seagen, Silverback Therapeutics, Strata Oncology, and UroGen Pharma; and has received institutional research funding from ALX Oncology, Acrivon, Bavarian Nordic, Bristol Myers Squibb, Clovis Oncology, Debiopharm Group, G1 Therapeutics, Gilead Sciences, GSK, Merck, Mirati Therapeutics, MSD, Pfizer, and QED Therapeutics. EG has served in consulting or advisory roles, received honoraria for speaker engagements, and received funding for continuous medical education from Adacap, Amgen, Angelini, Astellas, AstraZeneca, Bayer, Blueprint, Bristol Myers Squibb, Caris Life Sciences, Celgene, Clovis Oncology, Eisai, EUSA Pharma, Genetracer, Guardant Health, HRA-Pharma, Ipsen, ITM Radiopharma, Janssen, Lexicon, Lilly, Merck, MSD, NanoString Technologies, Natera, Novartis, Biosequence-OncoDNA, Palex, PharmaMar, Pierre Fabre, Pfizer, Roche, Sanofi Genzyme, Servier, Taiho, and Thermo Fisher Scientific; and has received research grants from Astellas, AstraZeneca, Lexicon Pharmaceuticals, and Pfizer. IDD is director and chair of the Australian and New Zealand Urogenital and Prostate Cancer Trials Group (ANZUP) and receives no remuneration; is supported in part by an Australian National Health and Medical Research Council Investigator Grant (2016274); and has served as a member or chair of advisory boards for the following companies within the past 5 years: Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, Ipsen, Janssen, Merck, MSD, Pfizer, Pio Therapeutics, Roche, and Xennials Therapeutics; all honoraria are paid directly to ANZUP. HHM has received honoraria from Merck and Pfizer; and has received research funding from Amgen, Apollomics, Arcus Biosciences, AVEO, Bristol Myers Squibb, Genentech, HUYA Bioscience International, Nektar, Prometheus, RevImmune, and Seagen. M-OG has received honoraria from Astellas Pharma, AstraZeneca, Bristol Myers Squibb, EUSA Pharma, Ipsen, Merck, MSD, and Pfizer; has served in a consulting or advisory role for Astellas Pharma, AstraZeneca, Bristol Myers Squibb, Eisai, EUSA Pharma, Merck, MSD, Pfizer, Roche Pharma AG, and Takeda; has received research funding from Bristol Myers Squibb and Intuitive; and has received travel, accommodations, and expenses from Bristol Myers Squibb and Merck. SG has served in consulting or advisory roles for AVEO, Gilead Sciences, Guardant Health, Loxo/Lilly, Merck, MSD, and Pfizer; has reported speakers services for Bristol Myers Squibb, Gilead Sciences, Janssen Oncology, and Seagen; has stock and other ownership interests in BioNTech, Moderna Therapeutics, and Nektar; and has received research funding from Bristol Myers Squibb, Gilead Sciences, Merck, MSD, Moderna, Pfizer, QED Therapeutics, Roche, and Seagen. PB has served in consulting or advisory roles for Amgen, Bristol Myers Squibb, Ipsen, Janssen-Cilag, Merck, MSD, and Pfizer; has received travel and accommodation expenses from Astellas Pharma, Bristol Myers Squibb, Ipsen, Janssen-Cilag, MSD, and Pfizer; and has received honoraria from Astellas Pharma, Bristol Myers Squibb, Ipsen, Janssen-Cilag, Merck, MSD, Novartis, Pfizer, and Seagen. CT has received honoraria from AAA, Astellas, AstraZeneca, Bristol Myers Squibb, Janssen, Ipsen, Merck, MSD, Pfizer, and Sanofi; has provided speaker services for Astellas, AstraZeneca, Bristol Myers Squibb, Ipsen, Janssen, MSD, and Sanofi; and has received institutional research funding from AstraZeneca and Sanofi. SG is an employee of Merck Healthcare KGaA, Darmstadt, Germany. SH is an employee of Pfizer. CNS has served in consulting or advisory roles for Astellas, AstraZeneca, Bayer, Bristol Myers Squibb/Medarex, Foundation Medicine, Gilead, IMPAC Medical Systems, Incyte, Janssen, Medscape, Merck, MSD, Pfizer, Roche, Sanofi Genzyme, and UroToday.
Figures


References
-
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
-
- Siegel R.L., Miller K.D., Wagle N.S., et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. - PubMed
-
- Singer S., Ziegler C., Schwalenberg T., et al. Quality of life in patients with muscle invasive and non-muscle invasive bladder cancer. Support Care Cancer. 2013;21(5):1383–1393. - PubMed
-
- Smith A.B., Jaeger B., Pinheiro L.C., et al. Impact of bladder cancer on health-related quality of life. BJU Int. 2018;121(4):549–557. - PubMed
-
- Yeung C., Dinh T., Lee J. The health economics of bladder cancer: an updated review of the published literature. Pharmacoeconomics. 2014;32(11):1093–1104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

